Page 433 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 433
396 Carraher’s Polymer Chemistry
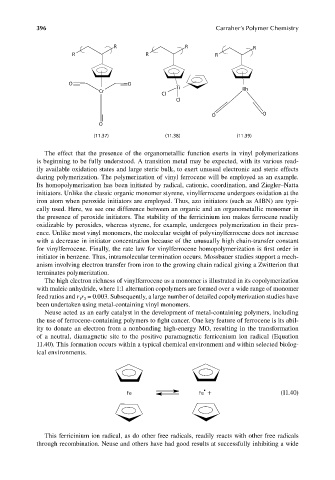
R R R
R R R
O O
Ti
Cr Rh
Cl
Cl
O O
O
(11.37) (11.38) (11.39)
The effect that the presence of the organometallic function exerts in vinyl polymerizations
is beginning to be fully understood. A transition metal may be expected, with its various read-
ily available oxidation states and large steric bulk, to exert unusual electronic and steric effects
during polymerization. The polymerization of vinyl ferrocene will be employed as an example.
Its homopolymerization has been initiated by radical, cationic, coordination, and Ziegler–Natta
initiators. Unlike the classic organic monomer styrene, vinylferrocene undergoes oxidation at the
iron atom when peroxide initiators are employed. Thus, azo initiators (such as AIBN) are typi-
cally used. Here, we see one difference between an organic and an organometallic monomer in
the presence of peroxide initiators. The stability of the ferricinium ion makes ferrocene readily
oxidizable by peroxides, whereas styrene, for example, undergoes polymerization in their pres-
ence. Unlike most vinyl monomers, the molecular weight of polyvinylferrocene does not increase
with a decrease in initiator concentration because of the unusually high chain-transfer constant
for vinylferrocene. Finally, the rate law for vinylferrocene homopolymerization is first order in
initiator in benzene. Thus, intramolecular termination occurs. Mossbauer studies support a mech-
anism involving electron transfer from iron to the growing chain radical giving a Zwitterion that
terminates polymerization.
The high electron richness of vinylferrocene as a monomer is illustrated in its copolymerization
with maleic anhydride, where 1:1 alternation copolymers are formed over a wide range of monomer
feed ratios and r r = 0.003. Subsequently, a large number of detailed copolymerization studies have
1 2
been undertaken using metal-containing vinyl monomers.
Neuse acted as an early catalyst in the development of metal-containing polymers, including
the use of ferrocene-containing polymers to fight cancer. One key feature of ferrocene is its abil-
ity to donate an electron from a nonbonding high-energy MO, resulting in the transformation
of a neutral, diamagnetic site to the positive paramagnetic ferricenium ion radical (Equation
11.40). This formation occurs within a typical chemical environment and within selected biolog-
ical environments.
Fe Fe + (11.40)
This ferricinium ion radical, as do other free radicals, readily reacts with other free radicals
through recombination. Neuse and others have had good results at successfully inhibiting a wide
9/14/2010 3:41:44 PM
K10478.indb 396 9/14/2010 3:41:44 PM
K10478.indb 396