Page 420 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 420
Organometallic and Inorganic–Organic Polymers 383
11.2 INORGANIC REACTION MECHANISMS
Many of the polymerizations and monomer syntheses are simply extensions of known inorganic,
organometallic, and organic reactions. The types and language used to describe inorganic–organic
reaction mechanisms are more diversified than those employed by classical organic chemists.
The majority of inorganic reactions can be placed into one of two broad classes—oxidation/
reduction (redox) reactions, including atom- and electron-transfer reactions and substitution reac-
tions. Terms such as inner sphere, outer sphere, and photo-related reactions are employed to describe
redox reactions. Such reactions are important in the synthesis of polymers and monomers and in the
use of metal-containing polymers as catalysts and in applications involving transfer of heat, elec-
tricity, and light. They will not be dealt with any appreciable extent in this chapter.
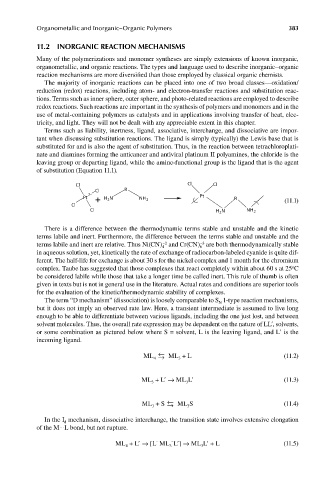
Terms such as liability, inertness, ligand, associative, interchange, and dissociative are impor-
tant when discussing substitution reactions. The ligand is simply (typically) the Lewis base that is
substituted for and is also the agent of substitution. Thus, in the reaction between tetrachloroplati-
nate and diamines forming the anticancer and antiviral platinum II polyamines, the chloride is the
leaving group or departing ligand, while the amine-functional group is the ligand that is the agent
of substitution (Equation 11.1).
Cl Cl Cl
Cl R
2–
Pt + H 2 N N H 2 Pt R (11.1)
Cl
Cl N
H 2 N H 2
There is a difference between the thermodynamic terms stable and unstable and the kinetic
terms labile and inert. Furthermore, the difference between the terms stable and unstable and the
–2
–3
terms labile and inert are relative. Thus Ni(CN) and Cr(CN) are both thermodynamically stable
4 6
in aqueous solution, yet, kinetically the rate of exchange of radiocarbon-labeled cyanide is quite dif-
ferent. The half-life for exchange is about 30 s for the nickel complex and 1 month for the chromium
o
complex. Taube has suggested that those complexes that react completely within about 60 s at 25 C
be considered labile while those that take a longer time be called inert. This rule of thumb is often
given in texts but is not in general use in the literature. Actual rates and conditions are superior tools
for the evaluation of the kinetic/thermodynamic stability of complexes.
The term “D mechanism” (dissociation) is loosely comparable to S 1-type reaction mechanisms,
N
but it does not imply an observed rate law. Here, a transient intermediate is assumed to live long
enough to be able to differentiate between various ligands, including the one just lost, and between
solvent molecules. Thus, the overall rate expression may be dependent on the nature of LL′, solvents,
or some combination as pictured below where S = solvent, L is the leaving ligand, and L′ is the
incoming ligand.
←
ML → ML + L (11.2)
4 3
ML + L′ → ML L′ (11.3)
3 3
←
ML + S → ML S (11.4)
3 3
In the I mechanism, dissociative interchange, the transition state involves extensive elongation
d
. . .
of the M L bond, but not rupture.
.. ..
ML + L′ → [L ML L′] → ML L′ + L (11.5)
4 3 3
K10478.indb 383 9/14/2010 3:41:28 PM
9/14/2010 3:41:28 PM
K10478.indb 383