Page 524 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 524
Rheology and Physical Tests 487
Eyring explained liquid flow using a random hole-filling model in which the holes (vacancies or
free volume) account for about 15% of the total volume at room temperature. The size of these holes
is similar to that for small molecules. The number of holes increases as the temperature increases,
and thus flow or hole filling is temperature dependent. Small molecules jump into the holes leaving
empty holes when their energy exceeds the activation energy. The activation energy for jumping or
moving into a hole is smaller, per individual unit, for linear molecules that fill the holes by succes-
sive correlated jumps of chain segments along the polymer chain. The jump frequency (ϕ) is gov-
erned by a segmental factor with both values related to molecular structure and temperature.
For convenience and simplicity, polymers have generally been considered to be isotropic in
which the principle force is shear stress. While such assumptions are acceptable for polymers at low
shear rates, they fail to account for stresses perpendicular to the plane of the shear stress, which are
encountered at high shear rates. For example, an extrudate such as the formation of a pipe or fi la-
ment, expands when it emerges from the die in what is called the Barus or Weissenberg effect or die
swell. This behavior is not explained by simple fl ow theories.
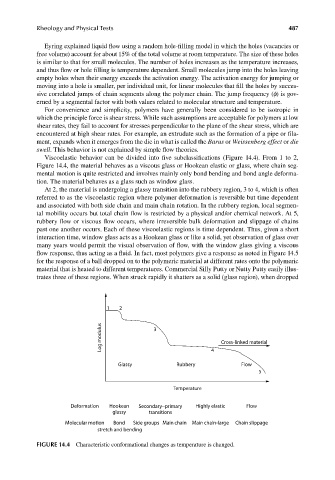
Viscoelastic behavior can be divided into fi ve subclassifications (Figure 14.4). From 1 to 2,
Figure 14.4, the material behaves as a viscous glass or Hookean elastic or glass, where chain seg-
mental motion is quite restricted and involves mainly only bond bending and bond angle deforma-
tion. The material behaves as a glass such as window glass.
At 2, the material is undergoing a glassy transition into the rubbery region, 3 to 4, which is often
referred to as the viscoelastic region where polymer deformation is reversible but time dependent
and associated with both side chain and main chain rotation. In the rubbery region, local segmen-
tal mobility occurs but total chain flow is restricted by a physical and/or chemical network. At 5,
rubbery flow or viscous flow occurs, where irreversible bulk deformation and slippage of chains
past one another occurs. Each of these viscoelastic regions is time dependent. Thus, given a short
interaction time, window glass acts as a Hookean glass or like a solid, yet observation of glass over
many years would permit the visual observation of flow, with the window glass giving a viscous
flow response, thus acting as a fluid. In fact, most polymers give a response as noted in Figure 14.5
for the response of a ball dropped on to the polymeric material at different rates onto the polymeric
material that is heated to different temperatures. Commercial Silly Putty or Nutty Putty easily illus-
trates three of these regions. When struck rapidly it shatters as a solid (glass region), when dropped
1 2
Log modulus 3 4 Cross-linked material
Glassy Rubbery Flow
5
Temperature
Deformation Hookean Secondary−primary Highly elastic Flow
glassy transitions
Molecular motion Bond Side groups Main chain Main chain-large Chain slippage
stretch and bending
FIGURE 14.4 Characteristic conformational changes as temperature is changed.
9/14/2010 3:42:31 PM
K10478.indb 487
K10478.indb 487 9/14/2010 3:42:31 PM