Page 525 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 525
488 Carraher’s Polymer Chemistry
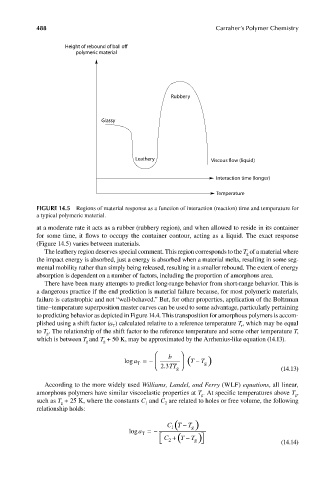
Height of rebound of ball off
polymeric material
Rubbery
Glassy
Leathery Viscous flow (liquid)
Interaction time (longer)
Temperature
FIGURE 14.5 Regions of material response as a function of interaction (reaction) time and temperature for
a typical polymeric material.
at a moderate rate it acts as a rubber (rubbery region), and when allowed to reside in its container
for some time, it flows to occupy the container contour, acting as a liquid. The exact response
(Figure 14.5) varies between materials.
The leathery region deserves special comment. This region corresponds to the T of a material where
g
the impact energy is absorbed, just a energy is absorbed when a material melts, resulting in some seg-
mental mobility rather than simply being released, resulting in a smaller rebound. The extent of energy
absorption is dependent on a number of factors, including the proportion of amorphous area.
There have been many attempts to predict long-range behavior from short-range behavior. This is
a dangerous practice if the end prediction is material failure because, for most polymeric materials,
failure is catastrophic and not “well-behaved.” But, for other properties, application of the Boltzman
time–temperature superposition master curves can be used to some advantage, particularly pertaining
to predicting behavior as depicted in Figure 14.4. This transposition for amorphous polymers is accom-
plished using a shift factor (a ) calculated relative to a reference temperature T, which may be equal
T
r
to T . The relationship of the shift factor to the reference temperature and some other temperature T,
g
which is between T and T + 50 K, may be approximated by the Arrhenius-like equation (14.13).
g
g
b
loga =− ( T − T )
T g
2.3TT g (14.13)
According to the more widely used Williams, Landel, and Ferry (WLF) equations, all linear,
amorphous polymers have similar viscoelastic properties at T . At specific temperatures above T ,
g
g
such as T + 25 K, where the constants C and C are related to holes or free volume, the following
2
1
g
relationship holds:
( −
CT T g )
1
loga =− )
T
2
C + ( T − T g (14.14)
K10478.indb 488 9/14/2010 3:42:31 PM
9/14/2010 3:42:31 PM
K10478.indb 488