Page 530 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 530
Rheology and Physical Tests 493
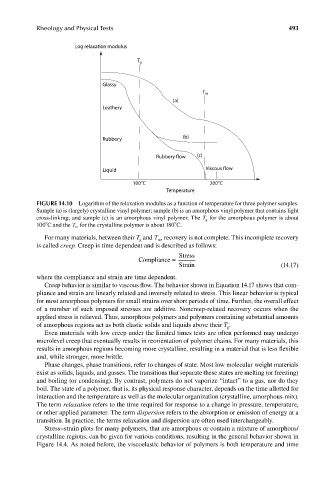
Log relaxation modulus
T g
Glassy
T m
(a)
Leathery
Rubbery (b)
Rubbery flow (c)
Liquid Viscous flow
100 C 200 C
Temperature
FIGURE 14.10 Logarithm of the relaxation modulus as a function of temperature for three polymer samples.
Sample (a) is (largely) crystalline vinyl polymer; sample (b) is an amorphous vinyl polymer that contains light
cross-linking; and sample (c) is an amorphous vinyl polymer. The T g for the amorphous polymer is about
o
o
100 C and the T m for the crystalline polymer is about 180 C.
For many materials, between their T and T , recovery is not complete. This incomplete recovery
m
g
is called creep. Creep is time dependent and is described as follows:
Stress
Compliance =
Strain (14.17)
where the compliance and strain are time dependent.
Creep behavior is similar to viscous flow. The behavior shown in Equation 14.17 shows that com-
pliance and strain are linearly related and inversely related to stress. This linear behavior is typical
for most amorphous polymers for small strains over short periods of time. Further, the overall effect
of a number of such imposed stresses are additive. Noncreep-related recovery occurs when the
applied stress is relieved. Thus, amorphous polymers and polymers containing substantial amounts
of amorphous regions act as both elastic solids and liquids above their T .
g
Even materials with low creep under the limited times tests are often performed may undergo
microlevel creep that eventually results in reorientation of polymer chains. For many materials, this
results in amorphous regions becoming more crystalline, resulting in a material that is less fl exible
and, while stronger, more brittle.
Phase changes, phase transitions, refer to changes of state. Most low molecular weight materials
exist as solids, liquids, and gasses. The transitions that separate these states are melting (or freezing)
and boiling (or condensing). By contrast, polymers do not vaporize “intact” to a gas, nor do they
boil. The state of a polymer, that is, its physical response character, depends on the time allotted for
interaction and the temperature as well as the molecular organization (crystalline, amorphous-mix).
The term relaxation refers to the time required for response to a change in pressure, temperature,
or other applied parameter. The term dispersion refers to the absorption or emission of energy at a
transition. In practice, the terms relaxation and dispersion are often used interchangeably.
Stress–strain plots for many polymers, that are amorphous or contain a mixture of amorphous/
crystalline regions, can be given for various conditions, resulting in the general behavior shown in
Figure 14.4. As noted before, the viscoelastic behavior of polymers is both temperature and time
9/14/2010 3:42:37 PM
K10478.indb 493 9/14/2010 3:42:37 PM
K10478.indb 493