Page 685 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 685
648 Carraher’s Polymer Chemistry
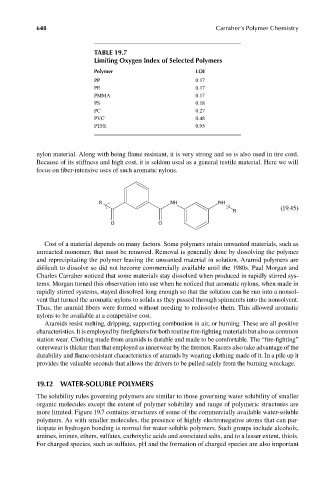
TABLE 19.7
Limiting Oxygen Index of Selected Polymers
Polymer LOI
PP 0.17
PE 0.17
PMMA 0.17
PS 0.18
PC 0.27
PVC 0.48
PTFE 0.95
nylon material. Along with being flame resistant, it is very strong and so is also used in tire cord.
Because of its stiffness and high cost, it is seldom used as a general textile material. Here we will
focus on fiber-intensive uses of such aromatic nylons.
R NH NH
(19.45)
R
O O
Cost of a material depends on many factors. Some polymers retain unwanted materials, such as
unreacted monomer, that must be removed. Removal is generally done by dissolving the polymer
and reprecipitating the polymer leaving the unwanted material in solution. Aramid polymers are
difficult to dissolve so did not become commercially available until the 1980s. Paul Morgan and
Charles Carraher noticed that some materials stay dissolved when produced in rapidly stirred sys-
tems. Morgan turned this observation into use when he noticed that aromatic nylons, when made in
rapidly stirred systems, stayed dissolved long enough so that the solution can be run into a nonsol-
vent that turned the aromatic nylons to solids as they passed through spinnerets into the nonsolvent.
Thus, the aramid fibers were formed without needing to redissolve them. This allowed aromatic
nylons to be available at a competitive cost.
Aramids resist melting, dripping, supporting combustion in air, or burning. These are all positive
characteristics. It is employed by fi refighters for both routine fi re-fighting materials but also as common
station wear. Clothing made from aramids is durable and made to be comfortable. The “fi re-fi ghting”
outerwear is thicker than that employed as innerwear by the firemen. Racers also take advantage of the
durability and flame-resistant characteristics of aramids by wearing clothing made of it. In a pile up it
provides the valuable seconds that allows the drivers to be pulled safely from the burning wreckage.
19.12 WATER-SOLUBLE POLYMERS
The solubility rules governing polymers are similar to those governing water solubility of smaller
organic molecules except the extent of polymer solubility and range of polymeric structures are
more limited. Figure 19.7 contains structures of some of the commercially available water-soluble
polymers. As with smaller molecules, the presence of highly electronegative atoms that can par-
ticipate in hydrogen bonding is normal for water-soluble polymers. Such groups include alcohols,
amines, imines, ethers, sulfates, carboxylic acids and associated salts, and to a lesser extent, thiols.
For charged species, such as sulfates, pH and the formation of charged species are also important
9/14/2010 3:44:05 PM
K10478.indb 648 9/14/2010 3:44:05 PM
K10478.indb 648