Page 689 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 689
652 Carraher’s Polymer Chemistry
in about an hour. Some fast cure systems will have about 20% cure in a few minutes and are almost
cured in about 2 h. Some instant cure systems are ready for use within seconds.
19.14 HYDROGELS
Hydrogels are simply water-filled gels. They are characterized by being hydrophilic yet not
completely soluble in water. Those hydrogels that are able to absorb large amounts of water are
referred to as superwater adsorbents.
Hydrogel structures may contain the hydrophilic units in the polymer backbone or as side chains.
Those polymers that contain the hydrophilic units in the backbone include acrylic, poly(vinyl alco-
hol), N-vinyl-2-pyrrolidione, and acrylamide-containing materials, derivatives of poly(ethylene
oxide), and ionomers and glycopolymers. Polymers that contain such functional groups as –OH,
–COOH, –SO H, –CONH can act as foundations for hydrogels. These polymers generally contain
3
2
some cross-linking that locks in a three-dimensional structure that prevents water solubility and
also helps retain water. The cross-links can be chemical or physical.
Applications of hydrogels include highly absorbent diapers based on poly(sodium acrylate), contact
lenses based on poly(2-hydroxyethyl methacrylate) (polyHEMA) and switches based on variations of
swelling of the hydrogels. A number of drug delivery systems have also been based on hydrogels.
19.15 EMERGING POLYMERS
A number of small-scale polymeric materials will continue to enter the marketplace on a regular basis.
These include biomaterials and electronics materials where the cost per pound is high and the pound-
age is low, generally well less than a 100 tons a year. These are materials that fulfi ll specifi c needs.
The number of new larger-scale giant molecules that enter the marketplace will be small. It has
been estimated that it takes about $1 billion to introduce and establish a new material. It is a daunting
task with no guarantee of success. In the past, new giant molecules could be introduced that offered
improvements in a number of areas and thus would attract a market share in a number of application
areas. Today, there are already a wide range of materials for most large-scale application areas that
compete for that particular market share so that it is difficult for any material to signifi cantly break
into any market area. A new material needs a “flagship” property that a particular market needs.
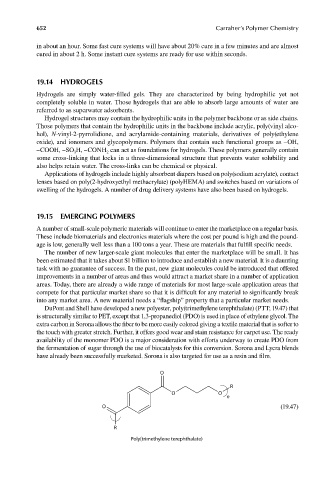
DuPont and Shell have developed a new polyester, poly(trimethylene terephthalate) (PTT; 19.47) that
is structurally similar to PET, except that 1,3-propanediol (PDO) is used in place of ethylene glycol. The
extra carbon in Sorona allows the fiber to be more easily colored giving a textile material that is softer to
the touch with greater stretch. Further, it offers good wear and stain resistance for carpet use. The ready
availability of the monomer PDO is a major consideration with efforts underway to create PDO from
the fermentation of sugar through the use of biocatalysts for this conversion. Sorona and Lycra blends
have already been successfully marketed. Sorona is also targeted for use as a resin and fi lm.
O
R
O O
n
O (19.47)
R
Poly(trimethylene terephthalate)
9/14/2010 3:44:07 PM
K10478.indb 652
K10478.indb 652 9/14/2010 3:44:07 PM