Page 687 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 687
650 Carraher’s Polymer Chemistry
over the adhesive to be sure that a ready supply of oxygen is present to prevent premature polymeriza-
tion from occurring. Even on application, the monomer is exposed to oxygen up until it is married in
such a manner as to cut off the supply of oxygen. At this point a hardener comes into play.
The hardener systems are complex. The most employed hardener system contains a three part
system—the actual radical-producing molecule, here a cumene hydroperoxide, an accelerator,
here N,N-dimethyl-p-toluidine, and saccharin, which acts as a metal complexing material and a
reducing agent for metal ion, here copper (Figure 19.9). The reaction between the saccharin and
N,N-dimethyl-p-toluidine consumes any remaining oxygen (Figure 19.9, top left). An aminal is
produced that dissolves surface metal ions reducing them to a lower oxidation number, here
+2
−
+1
Cu —> Cu + e (19.46)
+1
The Cu catalyzes the formation of radicals from the cumene hydroperoxide, which then begins
the polymerization of the TEGMA and TEGMA molecules with only one reactive end (Figure 19.10).
Those with two active ends result in the formation of cross-linked materials. A similar reaction
occurs with iron and several metals such as zinc, gold, silver, cadmium, magnesium, titanium, and
alloys that contain any of these metals.
The “aminal” reaction is cyclic ensuring the formation of an abundance of radicals that effect the
polymerization of TEGMA.
Because the vast majority of the monomers react at both ends, these adhesives are thermosets
and are brittle. Thus, such adhesives are not suitable for areas requiring flexibility. Because they
are thermosets, they are typically resistant to oils, water, and solvents. Thus, the adhesive plays two
roles—bonding and protection. These properties make anaerobic adhesives suitable for automotive
and truck motor mounts and electric engines.
R O
O CH 3
+
H C O O O
3
O O O CH 2
CH 2 O
O CH 2
H C O O O
3
C O O O CH 2
O
O
O 2
R
O CH 3
C O O O
H 3
O O O CH 2
O
O
O
O
TEGMA
R
Slow addition of TEGMA
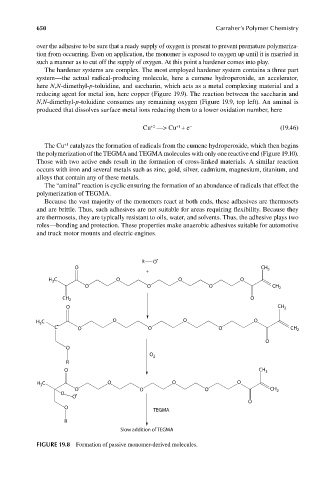
FIGURE 19.8 Formation of passive monomer-derived molecules.
9/14/2010 3:44:06 PM
K10478.indb 650
K10478.indb 650 9/14/2010 3:44:06 PM