Page 686 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 686
Selected Topics 649
Poly(vinyl alcohol) Poly(acrylic acid) Poly(sodium acrylate) Poly(ethylene oxide)
R R R R
R R R R O
OH
HO O O − + O
Na
R R R R
R NH R R R
NH 2 N O
N O
H 2
Polyethyleneimine Polyvinylamine Polyacrylamide Poly(vinyl pyrrolidone)
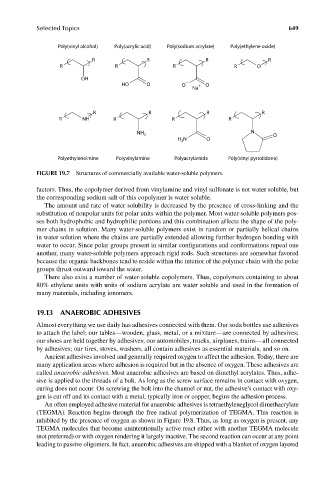
FIGURE 19.7 Structures of commercially available water-soluble polymers.
factors. Thus, the copolymer derived from vinylamine and vinyl sulfonate is not water soluble, but
the corresponding sodium salt of this copolymer is water soluble.
The amount and rate of water solubility is decreased by the presence of cross-linking and the
substitution of nonpolar units for polar units within the polymer. Most water-soluble polymers pos-
ses both hydrophobic and hydrophilic portions and this combination affects the shape of the poly-
mer chains in solution. Many water-soluble polymers exist in random or partially helical chains
in water solution where the chains are partially extended allowing further hydrogen bonding with
water to occur. Since polar groups present in similar configurations and conformations repeal one
another, many water-soluble polymers approach rigid rods. Such structures are somewhat favored
because the organic backbones tend to reside within the interior of the polymer chain with the polar
groups thrust outward toward the water.
There also exist a number of water-soluble copolymers. Thus, copolymers containing to about
80% ethylene units with units of sodium acrylate are water soluble and used in the formation of
many materials, including ionomers.
19.13 ANAEROBIC ADHESIVES
Almost everything we use daily has adhesives connected with them. Our soda bottles use adhesives
to attach the label; our tables—wooden, glass, metal, or a mixture—are connected by adhesives;
our shoes are held together by adhesives; our automobiles, trucks, airplanes, trains—all connected
by adhesives; our tires, stoves, washers, all contain adhesives as essential materials, and so on.
Ancient adhesives involved and generally required oxygen to affect the adhesion. Today, there are
many application areas where adhesion is required but in the absence of oxygen. These adhesives are
called anaerobic adhesives. Most anaerobic adhesives are based on dimethyl acrylates. Thus, adhe-
sive is applied to the threads of a bolt. As long as the screw surface remains in contact with oxygen,
curing does not occur. On screwing the bolt into the channel or nut, the adhesive’s contact with oxy-
gen is cut off and its contact with a metal, typically iron or copper, begins the adhesion process.
An often employed adhesive material for anaerobic adhesives is tetraethyleneglycol dimethacrylate
(TEGMA). Reaction begins through the free radical polymerization of TEGMA. This reaction is
inhibited by the presence of oxygen as shown in Figure 19.8. Thus, as long as oxygen is present, any
TEGMA molecules that become unintentionally active react either with another TEGMA molecule
(not preferred) or with oxygen rendering it largely inactive. The second reaction can occur at any point
leading to passive oligomers. In fact, anaerobic adhesives are shipped with a blanket of oxygen layered
9/14/2010 3:44:05 PM
K10478.indb 649 9/14/2010 3:44:05 PM
K10478.indb 649