Page 132 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 132
asymmetric reduction of ketones 119
Materials and equipment
. [Ru(allyl) (COD) ], 6 mg, 0.019 mmol, 0.02 eq
n
2
. (S)-(ÿ)-2,2 -Bis(diphenylphosphino)-1,1 -binaphthyl [(S)-BiNAP], 21 mg,
0
0
0.033 mmol, 0.035 eq
. Anhydrous acetone degassed (nitrogen) for 30 minutes, 30 mL
. Anhydrous methanol degassed (nitrogen) for 30 minutes, 30 mL
As the catalyst is very sensitive to oxygen, the solvents were degassed with
nitrogen just before use.
. Hydrobromic acid 48 %, 1.0 mL
. Hydrobromic acid solution 0.6 M prepared by mixing 9 mL of degassed
methanol and 1 mL of hydrobromic acid 48 %
. Methyl acetoacetate, 100 mL, 0.93 mmol
Methyl acetoacetate was dried with magnesium sulfate which was acti-
vated at 500 8C for 2 hours cooled under vacuum and stored under nitrogen.
. Petroleum ether, ethyl acetate, methanol
. Silica gel 60 (0.063±0.04 mm)
. p-Anisaldehyde
. 50 mL Schlenk tube
. Egg-shape magnetic stirrer bar
. Low-pressure hydrogenation apparatus fitted with a gas burette system to
measure the hydrogen consumed [36]
. Rotary evaporator
. Kugelrohr apparatus
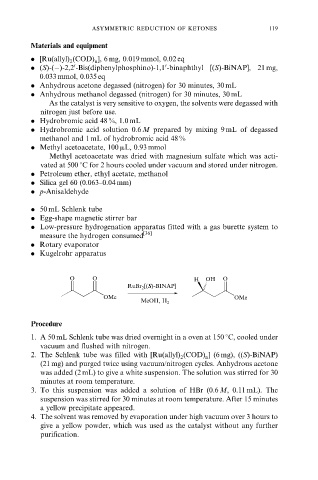
O O H OH O
RuBr 2 [(S)-BINAP]
OMe OMe
MeOH, H 2
Procedure
1. A 50 mL Schlenk tube was dried overnight in a oven at 150 8C, cooled under
vacuum and flushed with nitrogen.
2. The Schlenk tube was filled with [Ru(allyl) (COD) ] (6 mg), ((S)-BiNAP)
n
2
(21 mg) and purged twice using vacuum/nitrogen cycles. Anhydrous acetone
was added (2 mL) to give a white suspension. The solution was stirred for 30
minutes at room temperature.
3. To this suspension was added a solution of HBr (0.6 M, 0.11 mL). The
suspension was stirred for 30 minutes at room temperature. After 15 minutes
a yellow precipitate appeared.
4. The solvent was removed by evaporation under high vacuum over 3 hours to
give a yellow powder, which was used as the catalyst without any further
purification.