Page 131 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 131
118 hydrolysis, oxidation and reduction
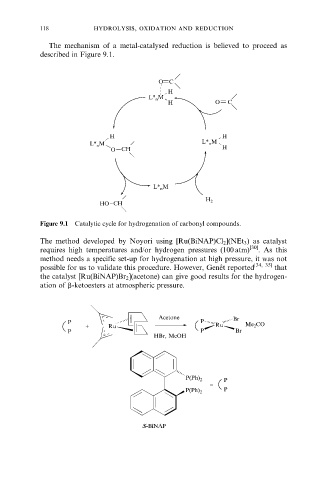
The mechanism of a metal-catalysed reduction is believed to proceed as
described in Figure 9.1.
O C
H
L* n M
H O C
H H
L* n M L* n M
O CH H
L* n M
H 2
HO CH
Figure 9.1 Catalytic cycle for hydrogenation of carbonyl compounds.
The method developed by Noyori using [Ru(BiNAP)Cl 2 ](NEt 3 ) as catalyst
requires high temperatures and/or hydrogen pressures (100 atm) [30] . As this
method needs a specific set-up for hydrogenation at high pressure, it was not
possible for us to validate this procedure. However, Gene Ãt reported [34, 35] that
the catalyst [Ru(BiNAP)Br 2 ](acetone) can give good results for the hydrogen-
ation of b-ketoesters at atmospheric pressure.
Acetone Br
P P Me 2 CO
+ Ru Ru
P P Br
HBr, MeOH
P
P(Ph) 2
=
P
P(Ph) 2
S-BiNAP