Page 126 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 126
112 hydrolysis, oxidation and reduction
Procedure
1. In a 10 mL round-bottomed flask equipped with a magnetic stirrer bar were
placed, under an argon atmosphere, anhydrous dichloromethane (2 mL) and
diethyl l-tartrate (0.21 mL).
2. The mixture was cooled to ÿ22 8C, then titanium(IV) isopropoxide (0.09 mL)
and hydroperoxide (228 mg) were added. Stirring was maintained for 10
minutes and racemic sulfoxide (110 mg solved in 1.5 mL of anhydrous dichlor-
omethane) added to the mixture.
3. The reaction was continued for 13 hours until completion [monitoring by
TLC (eluent: n-hexane±ethyl acetate, 5:1. Detector: UV lamp at 254 nm)
indicated complete consumption of the hydroperoxide].
4. After completion, water (1.5 mL) was added to the mixture and vigorous
stirring continued for 2 hours at room temperature. The resulting white gel
was diluted with ethyl acetate and filtered over a filter paper in a Bu Èchner
funnel. The solution was dried over sodium sulfate, filtered and concen-
trated using a rotary evaporator.
5. Purification of the crude mixture was performed by flash chromatography
to afford pure 5-(1-hydroxyethyl)-2-methyl-3-furoic acid 5a-cholestan-3b-yl
ester (86 % yield), 4-bromophenyl methyl sulfone (59 % yield) and (R)-4-
bromophenyl methyl sulfoxide (34 % yield).
1
The ee (>95 %) was determined on representative sample by H-NMR
analysis in the presence of R-(ÿ)-(3,5-dinitrobenzoyl)-a-methylbenzyl amine.
Stereoselection factors have been determined according to Kagan's equa-
[6]
tion .
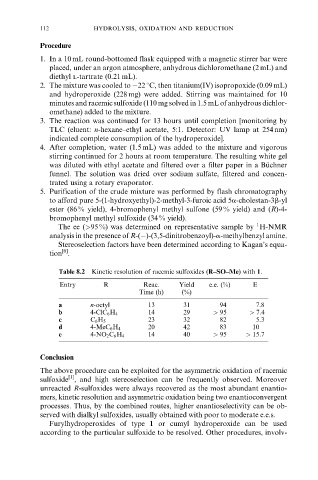
Table 8.2 Kinetic resolution of racemic sulfoxides (R±SO±Me) with 1.
Entry R Reac. Yield e.e. (%) E
Time (h) (%)
a n-octyl 13 31 94 7.8
b 4-ClC 6 H 4 14 29 > 95 > 7:4
c C 6 H 5 23 32 82 5.3
d 4-MeC 6 H 4 20 42 83 10
e 4-NO 2 C 6 H 4 14 40 > 95 > 15:7
Conclusion
The above procedure can be exploited for the asymmetric oxidation of racemic
[1]
sulfoxide , and high stereoselection can be frequently observed. Moreover
unreacted R-sulfoxides were always recovered as the most abundant enantio-
mers, kinetic resolution and asymmetric oxidation being two enantioconvergent
processes. Thus, by the combined routes, higher enantioselectivity can be ob-
served with dialkyl sulfoxides, usually obtained with poor to moderate e.e.s.
Furylhydroperoxides of type 1 or cumyl hydroperoxide can be used
according to the particular sulfoxide to be resolved. Other procedures, involv-