Page 121 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 121
asymmetric hydroxylation and aminohydroxylation 107
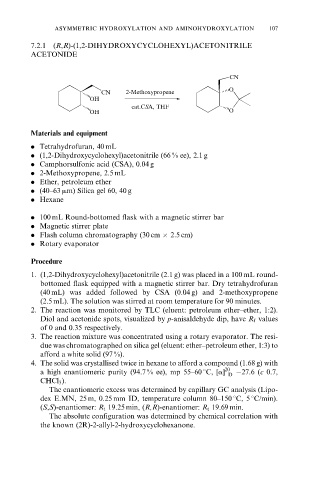
7.2.1 (R,R)-(1,2-DIHYDROXYCYCLOHEXYL)ACETONITRILE
ACETONIDE
CN
CN 2-Methoxypropene O
OH
cat.CSA, THF
OH O
Materials and equipment
. Tetrahydrofuran, 40 mL
. (1,2-Dihydroxycyclohexyl)acetonitrile (66 % ee), 2.1 g
. Camphorsulfonic acid (CSA), 0.04 g
. 2-Methoxypropene, 2.5 mL
. Ether, petroleum ether
. (40±63 mm) Silica gel 60, 40 g
. Hexane
. 100 mL Round-bottomed flask with a magnetic stirrer bar
. Magnetic stirrer plate
. Flash column chromatography (30 cm 2:5 cm)
. Rotary evaporator
Procedure
1. (1,2-Dihydroxycyclohexyl)acetonitrile (2.1 g) was placed in a 100 mL round-
bottomed flask equipped with a magnetic stirrer bar. Dry tetrahydrofuran
(40 mL) was added followed by CSA (0.04 g) and 2-methoxypropene
(2.5 mL). The solution was stirred at room temperature for 90 minutes.
2. The reaction was monitored by TLC (eluent: petroleum ether±ether, 1:2).
Diol and acetonide spots, visualized by p-anisaldehyde dip, have R f values
of 0 and 0.35 respectively.
3. The reaction mixture was concentrated using a rotary evaporator. The resi-
due was chromatographed on silica gel (eluent: ether±petroleum ether, 1:3) to
afford a white solid (97 %).
4. The solid was crystallised twice in hexane to afford a compound (1.68 g) with
a high enantiomeric purity (94.7 % ee), mp 55±60 8C, [a] 20 ÿ27.6 (c 0.7,
D
CHCl 3 ).
The enantiomeric excess was determined by capillary GC analysis (Lipo-
dex E.MN, 25 m, 0.25 mm ID, temperature column 80±150 8C, 5 8C/min).
(S,S)-enantiomer: R t 19.25 min, (R,R)-enantiomer: R t 19.69 min.
The absolute configuration was determined by chemical correlation with
the known (2R)-2-allyl-2-hydroxycyclohexanone.