Page 125 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 125
asymmetric sulfoxidation 111
Conclusion
The use of furylhydroperoxides [1] has facilitated an operationally simple
[2]
procedure, alternative to the one reported by Kagan . Oxidation takes place
rapidly and very high e.e.s have been obtained, especially in the case of aryl
methyl sulfides, while overoxidation to sulfone can be reduced to a great extent
(<3 %) under the proposed experimental conditions.
Modified catalytic procedures involving the employment of different
[4]
[3]
chiral ligands, such as binaphthol , 2,2,5,5-tetramethyl-3,4-hexanediol , 1,2-
diarylethane-1,2-diols [5] are often less selective and very variable values of e.e.s
can be observed.
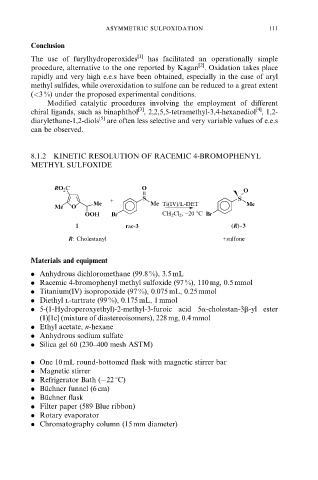
8.1.2 KINETIC RESOLUTION OF RACEMIC 4-BROMOPHENYL
METHYL SULFOXIDE
RO 2 C O
O
+ S S
Me Me Ti(IV)/L-DET Me
Me O
OOH Br CH 2 Cl 2 , −20 8C Br
1 rac-3 (R)-3
R: Cholestanyl +sulfone
Materials and equipment
. Anhydrous dichloromethane (99.8 %), 3.5 mL
. Racemic 4-bromophenyl methyl sulfoxide (97 %), 110 mg, 0.5 mmol
. Titanium(IV) isopropoxide (97 %), 0.075 mL, 0.25 mmol
. Diethyl l-tartrate (99 %), 0.175 mL, 1 mmol
. 5-(1-Hydroperoxyethyl)-2-methyl-3-furoic acid 5a-cholestan-3b-yl ester
(1)[1c] (mixture of diastereoisomers), 228 mg, 0.4 mmol
. Ethyl acetate, n-hexane
. Anhydrous sodium sulfate
. Silica gel 60 (230±400 mesh ASTM)
. One 10 mL round-bottomed flask with magnetic stirrer bar
. Magnetic stirrer
. Refrigerator Bath (ÿ22 8C)
. Bu Èchner funnel (6 cm)
. Bu Èchner flask
. Filter paper (589 Blue ribbon)
. Rotary evaporator
. Chromatography column (15 mm diameter)