Page 136 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 136
asymmetric reduction of ketones 123
Conclusion
This procedure offers a simple alternative for the reduction of b-ketoesters
which does not require the use of an autoclave or hydrogen. The reaction is
easily reproducible and leads to virtually quantitative yields of b-hydroxyesters
under mild conditions. The use of sterically hindered esters, i.e. iso-propyl or
tert-butyl b-ketoesters, significantly improves the catalytic activity, so that
reactions go to completion in a reasonable time at room temperature (see
below). When the reaction time is too long, transesterification may occur, giving
rise to a mixture of an alkyl b-hydroxyester and the corresponding iso-propyl b-
hydroxyester. Ephedrine as a chiral ligand affords modest to good enantiomeric
excesses according to the nature of the b-ketoester but is essential for good
activity of the catalytic system.
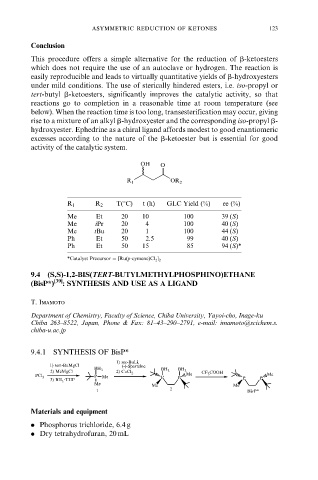
OH O
R 1 OR 2
R 1 R 2 T(8C) t (h) GLC Yield (%) ee (%)
Me Et 20 10 100 39 (S)
Me iPr 20 4 100 40 (S)
Me tBu 20 1 100 44 (S)
Ph Et 50 2.5 99 40 (S)
Ph Et 50 15 85 94 (S)*
*Catalyst Precursor [Ru(p-cymene)Cl 2 ] 2
9.4 (S,S)-1,2-BIS(TERT-BUTYLMETHYLPHOSPHINO)ETHANE
(BisP*) [39] : SYNTHESIS AND USE AS A LIGAND
T. Imamoto
Department of Chemistry, Faculty of Science, Chiba University, Yayoi-cho, Inage-ku
Chiba 263±8522, Japan, Phone & Fax: 81±43±290±2791, e-mail: imamoto@scichem.s.
chiba-u.ac.jp
9.4.1 SYNTHESIS OF BisP*
1) sec-BuLi,
1) tert-BuMgCl (-)-Sparteine
BH BH BH
2) MeMgCl 3 2) CuCl 2 3 3 CF COOH
PCl 3 P Me P P Me 3 Me
3) BH THF P P
3
Me Me Me
1 2 BisP*
Materials and equipment
. Phosphorus trichloride, 6.4 g
. Dry tetrahydrofuran, 20 mL