Page 146 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 146
asymmetric reduction of ketones 133
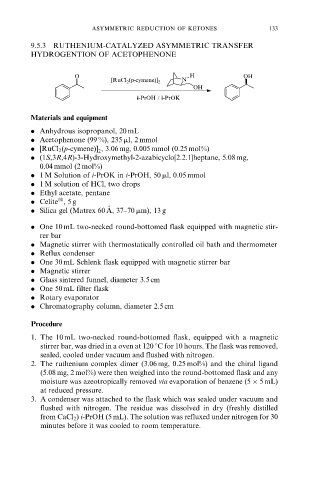
9.5.3 RUTHENIUM-CATALYZED ASYMMETRIC TRANSFER
HYDROGENTION OF ACETOPHENONE
O H OH
[RuCl 2 (p-cymene)] 2 N
OH
i-PrOH / i-PrOK
Materials and equipment
. Anhydrous isopropanol, 20 mL
. Acetophenone (99 %), 235 ml, 2 mmol
. [RuCl 2 (p-cymene)] , 3.06 mg, 0.005 mmol (0.25 mol%)
2
. (1S,3R,4R)-3-Hydroxymethyl-2-azabicyclo[2.2.1]heptane, 5.08 mg,
0.04 mmol (2 mol%)
. 1 M Solution of i-PrOK in i-PrOH, 50 ml, 0.05 mmol
. 1 M solution of HCl, two drops
. Ethyl acetate, pentane
1
. Celite , 5 g
Ê
. Silica gel (Matrex 60 A, 37±70 mm), 13 g
. One 10 mL two-necked round-bottomed flask equipped with magnetic stir-
rer bar
. Magnetic stirrer with thermostatically controlled oil bath and thermometer
. Reflux condenser
. One 30 mL Schlenk flask equipped with magnetic stirrer bar
. Magnetic stirrer
. Glass sintered funnel, diameter 3.5 cm
. One 50 mL filter flask
. Rotary evaporator
. Chromatography column, diameter 2.5 cm
Procedure
1. The 10 mL two-necked round-bottomed flask, equipped with a magnetic
stirrer bar, was dried in a oven at 120 8C for 10 hours. The flask was removed,
sealed, cooled under vacuum and flushed with nitrogen.
2. The ruthenium complex dimer (3.06 mg, 0.25 mol%) and the chiral ligand
(5.08 mg, 2 mol%) were then weighed into the round-bottomed flask and any
moisture was azeotropically removed via evaporation of benzene (5 5 mL)
at reduced pressure.
3. A condenser was attached to the flask which was sealed under vacuum and
flushed with nitrogen. The residue was dissolved in dry (freshly distilled
from CaCl 2 ) i-PrOH (5 mL). The solution was refluxed under nitrogen for 30
minutes before it was cooled to room temperature.