Page 144 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 144
asymmetric reduction of ketones 131
10. The major exo diastereoisomer was easily isolated from the crude reaction
mixture by column chromatography (deactivated silica gel [43] , pentane/
Et 2 O: 99/1 to 80/20) to give, 14.1 g, 55 % yield.
1 H NMR (200 MHz, CDCl 3 ): d 7.34±7.19 (5H, m), 6.36±6.33 (1H, m),
6.22±6.18 (1H, m), 4.37 (1H, s), 3.87 (2H, q, J 6.8 Hz), 3.10 (1H, q, J
6.5 Hz), 2.96 (1H, s), 2.27 (1H, s), 2.20 (1H, d, J 8.4 Hz), 1.48 (3H, d, J
6.5 Hz), 1.01 (3H, d, J 7.0 Hz), 1.00 (1H, d, J 8.4 Hz).
This procedure has been scaled up to provide 28 g of the major Diels±
Alder adduct.
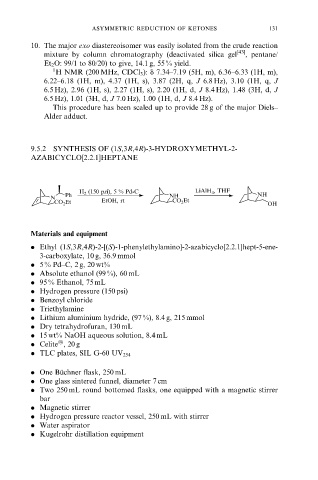
9.5.2 SYNTHESIS OF (1S,3R,4R)-3-HYDROXYMETHYL-2-
AZABICYCLO[2.2.1]HEPTANE
H 2 (150 psi), 5 % Pd-C LiAlH 4 , THF
Ph NH NH
N CO 2 Et
CO 2 Et EtOH, rt OH
Materials and equipment
. Ethyl (1S,3R,4R)-2-[(S)-1-phenylethylamino]-2-azabicyclo[2.2.1]hept-5-ene-
3-carboxylate, 10 g, 36.9 mmol
. 5 % Pd±C, 2 g, 20 wt%
. Absolute ethanol (99 %), 60 mL
. 95 % Ethanol, 75 mL
. Hydrogen pressure (150 psi)
. Benzoyl chloride
. Triethylamine
. Lithium aluminium hydride, (97 %), 8.4 g, 215 mmol
. Dry tetrahydrofuran, 130 mL
. 15 wt% NaOH aqueous solution, 8.4 mL
1
. Celite , 20 g
. TLC plates, SIL G-60 UV 254
. One Bu Èchner flask, 250 mL
. One glass sintered funnel, diameter 7 cm
. Two 250 mL round bottomed flasks, one equipped with a magnetic stirrer
bar
. Magnetic stirrer
. Hydrogen pressure reactor vessel, 250 mL with stirrer
. Water aspirator
. Kugelrohr distillation equipment