Page 147 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 147
134 hydrolysis, oxidation and reduction
4. Once the clear reddish solution of the catalyst had cooled to room tempera-
ture, it was transferred under a gentle stream of nitrogen to a Schlenk flask
containing a solution of the ketone (235 mL, 2 mmol) and potassium iso-
propoxide (0.05 mmol, 2.5 mol%) in i-PrOH (15 mL). The resulting solution
was then stirred for 1.5 hours at room temperature under nitrogen (moni-
1
tored by GC and/or H NMR).
5. After completion, the reaction was neutralized with a 1 M solution of HCl (2
drops) and concentrated in vacuo to give the crude product. After dilution
with ethyl acetate and removal of the catalyst by filtration over a thin pad of
Celite, the sample was analysed.
The ee of the alcohol (94 %) was determined by HPLC analysis (ChiralCel
OD-H) using a 254 nm UV detector and a flow rate of 0.5 mL/min of 5 % of
i-PrOH in hexane. (S)-a-Methylbenzyl alcohol (major): R t 20.65 min; (R)-a-
methylbenzyl alcohol (minor): R t 17.72 min.
6. Finally, the crude product was purified by flash chromatography (eluent:
pentane/ethyl acetate: 85/15) to afford 224.5 mg of pure alcohol (92 % yield).
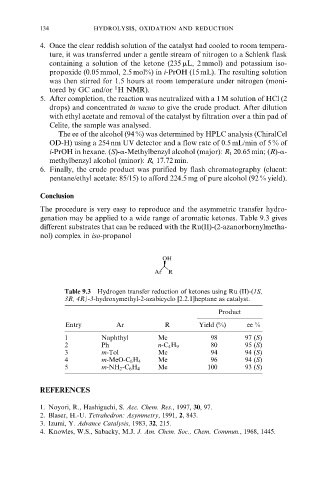
Conclusion
The procedure is very easy to reproduce and the asymmetric transfer hydro-
genation may be applied to a wide range of aromatic ketones. Table 9.3 gives
different substrates that can be reduced with the Ru(II)-(2-azanorbornylmetha-
nol) complex in iso-propanol
OH
Ar R
Table 9.3 Hydrogen transfer reduction of ketones using Ru (II)-(1S,
3R, 4R)-3-hydroxymethyl-2-azabicyclo [2.2.1]heptane as catalyst.
Product
Entry Ar R Yield (%) ee %
1 Naphthyl Me 98 97 (S)
2 Ph n-C 4 H 9 80 95 (S)
3 m-Tol Me 94 94 (S)
4 m-MeO-C 6 H 4 Me 96 94 (S)
5 m-NH 2 -C 6 H 4 Me 100 93 (S)
REFERENCES
1. Noyori, R., Hashiguchi, S. Acc. Chem. Res., 1997, 30, 97.
2. Blaser, H.-U. Tetrahedron: Asymmetry, 1991, 2, 843.
3. Izumi, Y. Advance Catalysis, 1983, 32, 215.
4. Knowles, W.S., Sabacky, M.J. J. Am. Chem. Soc., Chem. Commun., 1968, 1445.