Page 152 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 152
asymmetric reduction using bakers' yeast 139
1
NMR H (200 MHz, CDCl 3 ): d 4.18 (m, 1H, CH); 4.17 (q, J 7.15 Hz, 2H,
CH 2 CH 3 ); 3.17 (brs, 1H, exchangeable with D 2 O, OH); 2.45 (d, J 7 Hz, 2H,
CH 2 ); 1.29 (t, J 7.15 Hz, 3H, CH 2 CH 3 ); 1.23 (d, J 5.5 Hz, 2H, CHCH 3 ).
ÿ1
IR (Thin film, cm ): 3440, 2980, 1730, 1300.
Conclusion
In the original paper, the authors performed the reaction using commercially
available bakers' yeast from a supermarket or bakery. Initially a trial run using
similar quantities of Sigma dried yeast resulted in an extremely vigorous initial
fermentation, so the quantity of dry yeast was reduced by factor of 5. The
contributors assessed the enantiomeric excess of the alcohol by formation of
19
the ()-MTPA ester and examination of the F NMR spectrum. However, the
value obtained for the optical rotation was consistent with that reported in the
literature.
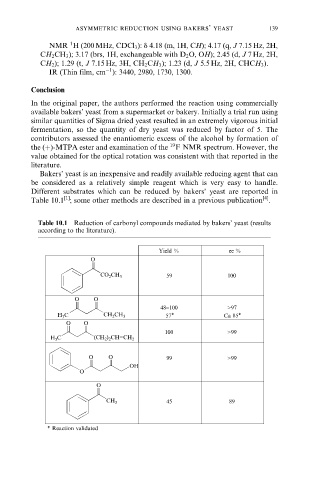
Bakers' yeast is an inexpensive and readily available reducing agent that can
be considered as a relatively simple reagent which is very easy to handle.
Different substrates which can be reduced by bakers' yeast are reported in
[1]
[4]
Table 10.1 ; some other methods are described in a previous publication .
Table 10.1 Reduction of carbonyl compounds mediated by bakers' yeast (results
according to the literature).
Yield % ee %
O
59 100
CO 2 CH 3
O O
48−100 >97
H 3 C CH 2 CH 3 57* Ca 85*
O O
100 >99
H 3 C (CH 2 ) 2 CH=CH 2
O O 99 >99
OH
O
O
45 89
CH 3
* Reaction validated