Page 142 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 142
asymmetric reduction of ketones 129
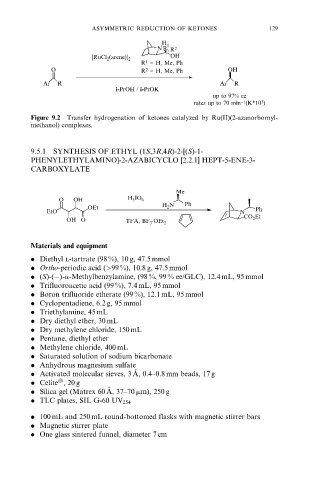
H
1
NR R 2
OH
[RuCl 2 (arene)] 2
R 1 = H, Me, Ph
O R 2 = H, Me, Ph OH
Ar R Ar R
i-PrOH / i-PrOK
up to 97% ee
rates up to 70 min −1 (K*10 3 )
Figure 9.2 Transfer hydrogenation of ketones catalyzed by Ru(II)(2-azanorbornyl-
methanol) complexes.
9.5.1 SYNTHESIS OF ETHYL (1S,3R,4R)-2-[(S)-1-
PHENYLETHYLAMINO]-2-AZABICYCLO [2.2.1] HEPT-5-ENE-3-
CARBOXYLATE
Me
O OH H 5 IO 6
OEt H 2 N Ph Ph
EtO N
CO 2 Et
OH O TFA, BF 3 OEt 2
.
Materials and equipment
. Diethyl l-tartrate (98 %), 10 g, 47.5 mmol
. Ortho-periodic acid (>99 %), 10.8 g, 47.5 mmol
. (S)-(ÿ)-a-Methylbenzylamine, (98 %, 99 % ee/GLC), 12.4 mL, 95 mmol
. Trifluoroacetic acid (99 %), 7.4 mL, 95 mmol
. Boron trifluoride etherate (99 %), 12.1 mL, 95 mmol
. Cyclopentadiene, 6.2 g, 95 mmol
. Triethylamine, 45 mL
. Dry diethyl ether, 30 mL
. Dry methylene chloride, 150 mL
. Pentane, diethyl ether
. Methylene chloride, 400 mL
. Saturated solution of sodium bicarbonate
. Anhydrous magnesium sulfate
Ê
. Activated molecular sieves, 3 A, 0.4±0.8 mm beads, 17 g
1
. Celite , 20 g
Ê
. Silica gel (Matrex 60 A, 37±70 mm), 250 g
. TLC plates, SIL G-60 UV 254
. 100 mL and 250 mL round-bottomed flasks with magnetic stirrer bars
. Magnetic stirrer plate
. One glass sintered funnel, diameter 7 cm