Page 166 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 166
asymmetric reduction using nonmetallic catalysts 153
8. The mixture was cooled to 0 8C with an ice-bath and then 2.1 mL of n-BuLi
(1.6 M in hexane) was added carefully via a syringe. The solution became
yellow.
9. The mixture was cooled to ÿ78 8C using an ethanol cooling bath. A
solution of benzophenone (682 mg) in dry tetrahydrofuran (3 mL) was
then added dropwise. The mixture was stirred for 2 hours at ÿ78 8C.
10. The reaction was followed by TLC (eluent: petroleum ether±ethyl acetate;
9:1). The benzophenone was UV active and stained yellow with perman-
ganate, R f 0.58. b-Hydroxysulphoximine was UV active and stained yellow
with p-anisaldehyde, R f 0.11.
11. The reaction was quenched with aqueous saturated solution of NH 4 Cl and
methanol (10:1, 2 mL). The mixture was stirred overnight at room tem-
perature and the solvent was evaporated under reduced pressure.
12. The alcohol was obtained by flash chromatography on silica gel eluting
with petroleum ether±ethyl acetate (9:1) to eliminate the benzophenone and
then with an eluant ratio 6:4, giving (SS)-1,1-diphenyl-2-(S-phenylsulfoni-
midoyl)-ethanol (790 mg, 2.3 mmol, 77 %).
. The yield of the reaction is variable (33±77 %), especially if the reaction
is not carried out under strictly anhydrous conditions or if the flash
chromatography takes an excessive amount of time.
. 1 H NMR(200 MHz, CDCl 3 ): d 7.6±7.09 (m, 15H, Ph); 4.11 (s, 2H, CH 2 );
2.96 (br s, 1H, NH).
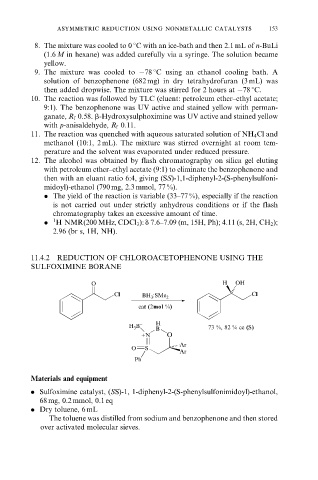
11.4.2 REDUCTION OF CHLOROACETOPHENONE USING THE
SULFOXIMINE BORANE
O H OH
Cl Cl
BH 3 :SMe 2
cat (2mol %)
− H
H 3 B 73 %, 82 % ee (S)
B
+N O
Ar
O S
Ar
Ph
Materials and equipment
. Sulfoximine catalyst, (SS)-1, 1-diphenyl-2-(S-phenylsulfonimidoyl)-ethanol,
68 mg, 0.2 mmol, 0.1 eq
. Dry toluene, 6 mL
The toluene was distilled from sodium and benzophenone and then stored
over activated molecular sieves.