Page 168 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 168
asymmetric reduction using nonmetallic catalysts 155
1
H NMR(200 MHz, CDCl 3 ): d 7.39±7.31 (m, 5H, Ph); 4.88 (ddd, J 8.8 Hz,
J 3.3 Hz, J 3.3 Hz, 1H, CH); 3.74 (dd, J 3.3 Hz, J 11.5 Hz, 1H, CH a H b ); 3.70
(dd, J 8.8 Hz, J 11.8 Hz, 1H, CH a H b ); 2.78 (br s, 1H, OH).
Conclusion
To obtain a good enantiomeric excess, the ligand synthesis and the reduction
reaction need to be carried out under strictly anhydrous conditions. The
addition of the substrate needs to be as slow as possible. Table 11.3 gives
some examples of the different substrates that can be reduced by the hydro-
xysulfoximine-borane catalyst described. Other examples are given in the com-
parative Table 11.4. Concerning the synthesis of the catalyst, the yield can
dramatically decrease if the reaction conditions are not strictly anhydrous.
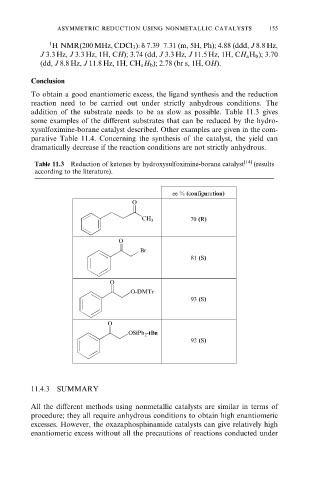
Table 11.3 Reduction of ketones by hydroxysulfoximine-borane catalyst [14] (results
according to the literature).
ee % (configuration)
O
70 (R)
CH 3
O
Br
81 (S)
O
O-DMTr
93 (S)
O
OSiPh 2 -tBu
92 (S)
11.4.3 SUMMARY
All the different methods using nonmetallic catalysts are similar in terms of
procedure; they all require anhydrous conditions to obtain high enantiomeric
excesses. However, the oxazaphosphinamide catalysts can give relatively high
enantiomeric excess without all the precautions of reactions conducted under