Page 170 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 170
asymmetric reduction using nonmetallic catalysts 157
11.5 ASYMMETRIC REDUCTION OF BROMOKETONE CATALYZED
BY CIS-AMINOINDANOL OXAZABOROLIDINE
Chris H. Senanayake, H. Scott Wilkinson and Gerald J. Tanoury
Chemical Research and Development, Sepracor Inc., 111 Locke Drive, Marlbrough, MA
01752, USA
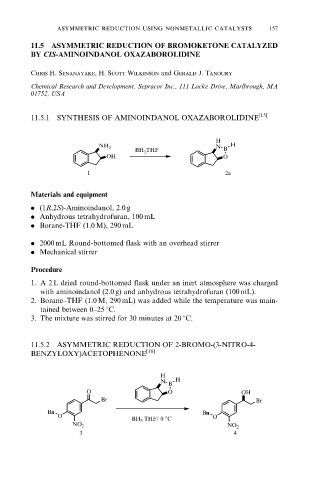
11.5.1 SYNTHESIS OF AMINOINDANOL OXAZABOROLIDINE [15]
H
NH 2 N H
BH 3 THF B
OH O
1 2a
Materials and equipment
. (1R,2S)-Aminoindanol, 2.0 g
. Anhydrous tetrahydrofuran, 100 mL
. Borane-THF (1.0 M), 290 mL
. 2000 mL Round-bottomed flask with an overhead stirrer
. Mechanical stirrer
Procedure
1. A 2 L dried round-bottomed flask under an inert atmosphere was charged
with aminoindanol (2.0 g) and anhydrous tetrahydrofuran (100 mL).
2. Borane±THF (1.0 M, 290 mL) was added while the temperature was main-
tained between 0±25 8C.
3. The mixture was stirred for 30 minutes at 20 8C.
11.5.2 ASYMMETRIC REDUCTION OF 2-BROMO-(3-NITRO-4-
BENZYLOXY)ACETOPHENONE [16]
H
N H
B
O O OH
Br Br
Bn Bn
O O
BH 3 THF/ 0 8C
NO 2 NO 2
3 4