Page 172 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 172
asymmetric reduction using nonmetallic catalysts 159
. Magnetic stirrer
. Buchner funnel
Procedure
1. 2-Bromo-(3-nitro-4-benzyloxyphenyl) ethanol (93 % ee, 48 g) and 100 mL of
toluene were placed in a 500 mL flask. The mixture was warmed until all the
alcohol dissolved. The mixture was cooled to 5 8C and stirred for 1 hour.
2. Heptane (100 mL) was slowly added to the stirring mixture and that solution
was stirred for 1 hour at 5 8C.
3. The slurry was filtered and the solid washed with heptane (25 mL).
4. The solid was dried in a vacuum oven to yield 45 g of 2-bromo-(3-nitro-4-
benzyloxyphenyl) ethanol (>99 % ee).
1 H NMR (300 MHz DMSO-d6): d 7.88 (m, 1H), 7.65 (d, 1H), 7.3±7.5 (m,
6 H), 6.01 (d, 1H), 5.35 (s, 2 H), 4.83 (m, 1H), 3.64 (ddd, 2H).
13 C NMR (MHz DMSO-d6): d 151.94, 140.15, 135.52, 133.24, 131.73,
128.95, 128.51, 127.51, 123.62, 115.47, 72.40, 71.45, 39.75.
IR: (KBr): 3381 (OH), 3091, 3067, 2961, 2893 (C±H), 1532, 1296, 1026,
ÿ1
729 cm .
11.5.3 CONCLUSIONS
This procedure has been developed through the evaluation of several reaction
parameters (catalyst, temperature, borane source, additives) and has been
successfully used on large scale. The chemical purity of the product is excellent
and the enantiomeric purity of the product can be increased by crystallizing
from toluene/heptane.
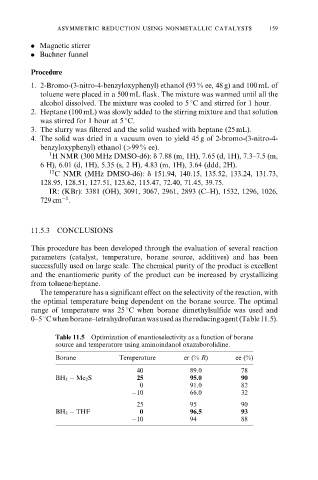
The temperature has a significant effect on the selectivity of the reaction, with
the optimal temperature being dependent on the borane source. The optimal
range of temperature was 25 8C when borane±dimethylsulfide was used and
0±5 8Cwhenborane±tetrahydrofuranwasusedasthereducingagent(Table11.5).
Table 11.5 Optimization of enantioselectivity as a function of borane
source and temperature using aminoindanol oxazaborolidine.
Borane Temperature er (% R) ee (%)
40 89.0 78
BH 3 ÿ Me 2 S 25 95.0 90
0 91.0 82
ÿ10 66.0 32
25 95 90
BH 3 ÿ THF 0 96.5 93
ÿ10 94 88