Page 177 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 177
164 hydrolysis, oxidation and reduction
3. After being stirred for 2 hours at room temperature, 10 M borane±dimethyl-
sulfide complex (2.0 mL) was added. The mixture was heated under reflux
for 65 hours. The resulting mixture was cooled to room temperature and
cautiously transferred into 2 N hydrochloric acid (10 mL) in a 200 mL
round-bottomed flask equipped with a magnetic stirrer bar using diethyl
ether (10 mL).
4. After being stirred for 1 hour at 60 8C, the mixture was cooled to room
temperature and then made basic with sodium hydroxide (1.4 g). Benzoyl
chloride (0.70 g) was added, and the mixture was stirred for 1 hour at room
temperature and subsequently stirred for 2 hour at 60 8C with methanol
(10 mL).
5. The organic solvents were removed under reduced pressure using a rotary
evaporator. The residue was transferred into a separating funnel with meth-
ylene chloride±tetrahydrofuran (2:1, 40 mL) and the phases were separated.
The aqueous layer was extracted with methylene chloride±tetrahydrofuran
(2:1, 2 40 mL). The combined organic layers were dried over magnesium
sulfate, filtered and concentrated using a rotary evaporator.
6. The residue was purified by silica gel column chromatography using meth-
ylene chloride±methanol (50:1 ! 30:1) as an eluent to give a white solid N-
benzoylsphingamine (0.93 g, 92 %) as a mixture of diastereomers.
The anti/syn ratio (13:87) and the respective ee (anti 75 %, syn 89 %) were
1
determined by HPLC (YMC Chiral NEA chiral column (i.d. 4.6 250 mm)
and Chiralcel OJ-R chiral column (i.d. 4.6 150 mm) connected in series, flow
0.3 mL/min, eluent acetonitrile±water 3:7, detection UV 254 nm); 65.6 min for
(2S, 3R)-isomer, 68.0 min for (2R, 3S)-isomer, 71.8 min for (2R,3R)-isomer,
74.2 min for (2S,3S)-isomer.
1 H NMR (270 MHz, CDCl 3 ) for syn isomer d 0.88 (t, J 6.7 Hz, 3H), 1.25
(br, 26H), 1.50 (m, 2H), 3.32 (br, 2H), , 3.88 (m, 2H), 4.03±4.14 (m, 2H), 6.97
(d, J 8.6 Hz, 1H), 7.40 (t, J 7.3 Hz, 2H), 7.48 (d, J 7.3 Hz, 1H), 7.78 (d, J
7.3 Hz, 2H).
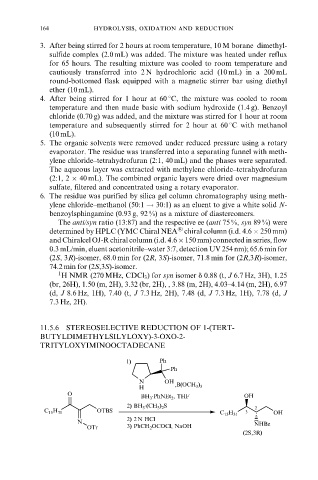
11.5.6 STEREOSELECTIVE REDUCTION OF 1-(TERT-
BUTYLDIMETHYLSILYLOXY)-3-OXO-2-
TRITYLOXYIMINOOCTADECANE
1) Ph
Ph
N OH
H ,B(OCH 3 ) 3
O .
BH 3 PhNEt 2 , THF OH
.
2) BH 3 (CH 3 ) 2 S 2
OTBS
C 15 H 31 3 OH
C 15 H 31
2) 2 N HCl
N NHBz
OTr 3) PhCH 2 OCOCl, NaOH
(2S,3R)