Page 180 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 180
asymmetric reduction using nonmetallic catalysts 167
were combined and washed with brine, dried over magnesium sulfate and
concentrated to give a white solid. The solid was purified by crystallization
using ethyl acetate to give the product as white crystals (10 g, 87 %).
1 H NMR (400 MHz, CDCl 3 ): d 8.66 (d, J 4.8 Hz, 1H), 8.10 (d, J 8 Hz,
1H), 8.00 (m, 1H), 7.57 (m, 1H), 7.41 (dd, J 5.2, 7.6 Hz, 1H), 7.3±7.2 (m,
4H), 5.69 (d, J 8 Hz, 1H), 4.95 (dd, J 4.8, 9.7 Hz, 1H), 4.27 (m, 1H), 3.06 (dd,
J 5.6, 16.7 Hz, 1H), 2.94 (d, J 16.6 Hz, 1H).
13 C NMR (100 MHz, CDCl 3 ): d 158.8, 149.2, 140.0, 139.4, 139.1, 128.7,
127.3, 127.3, 125.3, 124.9, 122.5, 72.1, 62.3, 38.8.
Rotation was recorded on a JASCO-DIP-370 instrument: [a] 25 ÿ 37:0 (c
D
1.0, CHCl 3 ).
Analysis calculated for C 14 H 14 N 2 O 3 S: C, 57.92, H, 4.86, N, 9.65, Found:
C, 57.67, H, 4.57, N, 9.65.
The quality of the ligand can be determined by performing an asym-
metric reduction reaction on prochiral ketones according to the following
procedure.
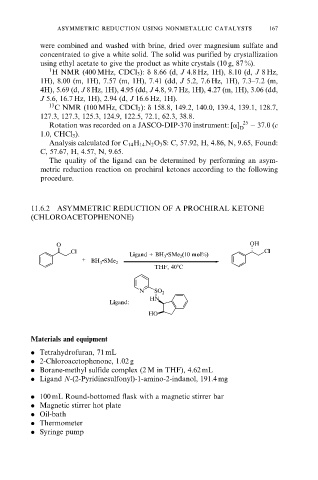
11.6.2 ASYMMETRIC REDUCTION OF A PROCHIRAL KETONE
(CHLOROACETOPHENONE)
O OH
Cl Cl
Ligand + BH 3 •SMe 2 (10 mol%)
+
BH 3 •SMe 2
THF, 408C
N SO 2
HN
Ligand:
HO
Materials and equipment
. Tetrahydrofuran, 71 mL
. 2-Chloroacetophenone, 1.02 g
. Borane-methyl sulfide complex (2 M in THF), 4.62 mL
. Ligand N-(2-Pyridinesulfonyl)-1-amino-2-indanol, 191.4 mg
. 100 mL Round-bottomed flask with a magnetic stirrer bar
. Magnetic stirrer hot plate
. Oil-bath
. Thermometer
. Syringe pump