Page 176 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 176
asymmetric reduction using nonmetallic catalysts 163
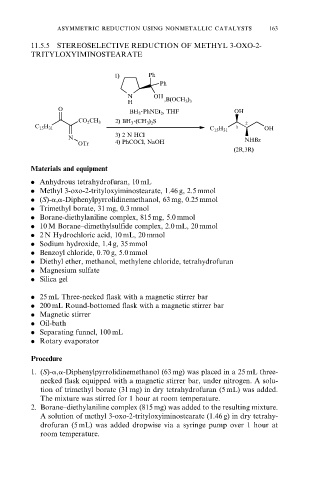
11.5.5 STEREOSELECTIVE REDUCTION OF METHYL 3-OXO-2-
TRITYLOXYIMINOSTEARATE
1) Ph
Ph
N OH
H ,B(OCH 3 ) 3
.
O BH 3 PhNEt 2 , THF OH
.
2) BH 3 (CH 3 ) 2 S
CO 2 CH 3
2
C 15 H 31 3 OH
C 15 H 31
3) 2 N HCl
N NHBz
OTr 4) PhCOCl, NaOH
(2R,3R)
Materials and equipment
. Anhydrous tetrahydrofuran, 10 mL
. Methyl 3-oxo-2-trityloxyiminostearate, 1.46 g, 2.5 mmol
. (S)-a,a-Diphenylpyrrolidinemethanol, 63 mg, 0.25 mmol
. Trimethyl borate, 31 mg, 0.3 mmol
. Borane-diethylaniline complex, 815 mg, 5.0 mmol
. 10 M Borane±dimethylsulfide complex, 2.0 mL, 20 mmol
. 2 N Hydrochloric acid, 10 mL, 20 mmol
. Sodium hydroxide, 1.4 g, 35 mmol
. Benzoyl chloride, 0.70 g, 5.0 mmol
. Diethyl ether, methanol, methylene chloride, tetrahydrofuran
. Magnesium sulfate
. Silica gel
. 25 mL Three-necked flask with a magnetic stirrer bar
. 200 mL Round-bottomed flask with a magnetic stirrer bar
. Magnetic stirrer
. Oil-bath
. Separating funnel, 100 mL
. Rotary evaporator
Procedure
1. (S)-a,a-Diphenylpyrrolidinemethanol (63 mg) was placed in a 25 mL three-
necked flask equipped with a magnetic stirrer bar, under nitrogen. A solu-
tion of trimethyl borate (31 mg) in dry tetrahydrofuran (5 mL) was added.
The mixture was stirred for 1 hour at room temperature.
2. Borane±diethylaniline complex (815 mg) was added to the resulting mixture.
A solution of methyl 3-oxo-2-trityloxyiminostearate (1.46 g) in dry tetrahy-
drofuran (5 mL) was added dropwise via a syringe pump over 1 hour at
room temperature.