Page 161 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 161
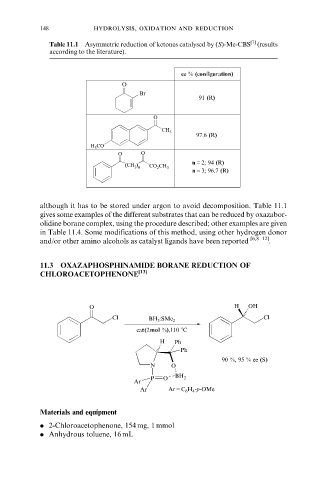
148 hydrolysis, oxidation and reduction
Table 11.1 Asymmetric reduction of ketones catalysed by (S)-Me-CBS [7] (results
according to the literature).
ee % (configuration)
O
Br
91 (R)
O
CH 3
97.6 (R)
H 3 CO
O O
n = 2; 94 (R)
(CH 2 ) n
CO 2 CH 3
n = 3; 96.7 (R)
although it has to be stored under argon to avoid decomposition. Table 11.1
gives some examples of the different substrates that can be reduced by oxazabor-
olidine borane complex, using the procedure described; other examples are given
in Table 11.4. Some modifications of this method, using other hydrogen donor
and/or other amino alcohols as catalyst ligands have been reported [6,8±12] .
11.3 OXAZAPHOSPHINAMIDE BORANE REDUCTION OF
CHLOROACETOPHENONE [13]
O H OH
Cl BH 3 :SMe 2 Cl
cat(2mol %),110 8C
H Ph
Ph
90 %, 95 % ee (S)
N O
P O BH 2
Ar
Ar Ar = C 6 H 4 -p-OMe
Materials and equipment
. 2-Chloroacetophenone, 154 mg, 1 mmol
. Anhydrous toluene, 16 mL