Page 159 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 159
146 hydrolysis, oxidation and reduction
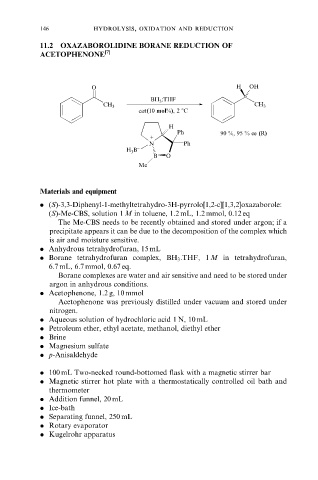
11.2 OXAZABOROLIDINE BORANE REDUCTION OF
ACETOPHENONE [7]
O H OH
BH 3 :THF
CH 3 CH 3
cat(10 mol%), 2 8C
H
Ph 90 %, 95 % ee (R)
+
N Ph
H 3 B −
B O
Me
Materials and equipment
. (S)-3,3-Diphenyl-1-methyltetrahydro-3H-pyrrolo[1,2-c][1,3,2]oxazaborole:
(S)-Me-CBS, solution 1 M in toluene, 1.2 mL, 1.2 mmol, 0.12 eq
The Me-CBS needs to be recently obtained and stored under argon; if a
precipitate appears it can be due to the decomposition of the complex which
is air and moisture sensitive.
. Anhydrous tetrahydrofuran, 15 mL
. Borane tetrahydrofuran complex, BH 3 :THF, 1 M in tetrahydrofuran,
6.7 mL, 6.7 mmol, 0.67 eq.
Borane complexes are water and air sensitive and need to be stored under
argon in anhydrous conditions.
. Acetophenone, 1.2 g, 10 mmol
Acetophenone was previously distilled under vacuum and stored under
nitrogen.
. Aqueous solution of hydrochloric acid 1 N, 10 mL
. Petroleum ether, ethyl acetate, methanol, diethyl ether
. Brine
. Magnesium sulfate
. p-Anisaldehyde
. 100 mL Two-necked round-bottomed flask with a magnetic stirrer bar
. Magnetic stirrer hot plate with a thermostatically controlled oil bath and
thermometer
. Addition funnel, 20 mL
. Ice-bath
. Separating funnel, 250 mL
. Rotary evaporator
. Kugelrohr apparatus