Page 222 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 222
210 hydrolysis, oxidation and reduction
1
H-NMR (300 MHz, CDCl 3 ): d 7.25±7.18 (m, 3H), 7.04±7.00 (m, 2H),
5.96 (d, J 7.1 Hz, 1 H), 4.85±4.78 (m, 1 H), 3.65 (s, 3 H), 3.11±2.97 (m, 2H),
1.90 (s, 3 H).
13 C-NMR (75 MHz, CDCl 3 ): d 172.07, 169.52, 135.85, 129.18, 128.51,
127.06, 53.10, 52.21, 37.83, 23.02.
Conclusion
The straightforward synthesis of the diamino FERRIPHOS ligand offers a
convenient access to this class of ferrocenyl ligands [32] and makes this ligand
well suited for applications in asymmetric hydrogenation.
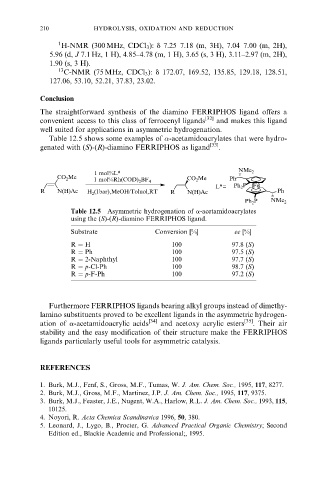
Table 12.5 shows some examples of -acetamidoacrylates that were hydro-
genated with (S)-(R)-diamino FERRIPHOS as ligand [33] .
1 mol%L* NMe 2
CO 2 Me CO 2 Me Ph
1 mol%Rh(COD) 2 BF 4
L*= Ph 2 P Fe
R N(H)Ac H 2 (1bar),MeOH/Toluol,RT R N(H)Ac Ph
Ph 2 P NMe 2
Table 12.5 Asymmetric hydrogenation of a-acetamidoacrylates
using the (S)-(R)-diamino FERRIPHOS ligand.
Substrate Conversion [%] ee [%]
R H 100 97.8 (S)
R Ph 100 97.5 (S)
R 2-Naphthyl 100 97.7 (S)
R p-Cl-Ph 100 98.7 (S)
R p-F-Ph 100 97.2 (S)
Furthermore FERRIPHOS ligands bearing alkyl groups instead of dimethy-
lamino substituents proved to be excellent ligands in the asymmetric hydrogen-
ation of a-acetamidoacrylic acids [34] and acetoxy acrylic esters [35] . Their air
stability and the easy modification of their structure make the FERRIPHOS
ligands particularly useful tools for asymmetric catalysis.
REFERENCES
1. Burk, M.J., Fenf, S., Gross, M.F., Tumas, W. J. Am. Chem. Soc., 1995, 117, 8277.
2. Burk, M.J., Gross, M.F., Martinez, J.P. J. Am. Chem. Soc., 1995, 117, 9375.
3. Burk, M.J., Feaster, J.E., Nugent, W.A., Harlow, R.L. J. Am. Chem. Soc., 1993, 115,
10125.
4. Noyori, R. Acta Chemica Scandinavica 1996, 50, 380.
5. Leonard, J., Lygo, B., Procter, G. Advanced Practical Organic Chemistry; Second
Edition ed., Blackie Academic and Professional;, 1995.