Page 218 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 218
206 hydrolysis, oxidation and reduction
pyridine (4 mL) and acetic anhydride (2 mL) was added. The reaction mix-
ture was stirred for 12 hours at room temperature.
2. All volatile material was evaporated under vacuum (1 mmHg, 3 hours)
yielding (S,S)-1,1 -bis(a-acetoxyphenylmethyl)ferrocene in quantitative
0
yield as a brown oil.
1 H-NMR (300 MHz, CDCl 3 ): d 7.30±7.20 (m, 10 H), 6.57 (s, 2 H), 4.25±
4.23 (m, 2 H), 4.03±4.02 (m, 2 H), 3.98±3.97 (m, 2 H), 3.85±3.84 (m, 2 H),
2.04 (s, 6 H).
13 C-NMR (75 MHz, CDCl 3 ): d 169.91, 139.97, 128.27, 128.03, 127.17,
88.46, 74.06, 69.32, 69.27, 68.58, 68.37, 21.26.
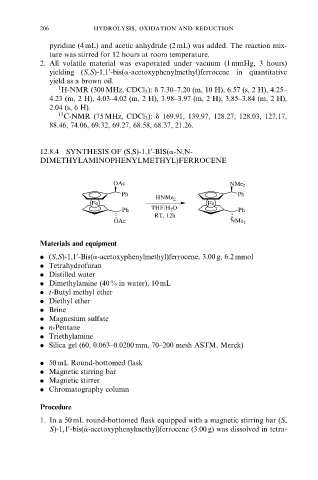
12.8.4 SYNTHESIS OF (S,S)-1,1 -BIS(a-N,N-
0
DIMETHYLAMINOPHENYLMETHYL)FERROCENE
OAc NMe 2
Ph Ph
HNMe 2
Fe Fe
2
Ph THF/H O Ph
RT, 12h
OAc NMe 2
Materials and equipment
. (S,S)-1,1 -Bis(a-acetoxyphenylmethyl)ferrocene, 3.00 g, 6.2 mmol
0
. Tetrahydrofuran
. Distilled water
. Dimethylamine (40 % in water), 10 mL
. t-Butyl methyl ether
. Diethyl ether
. Brine
. Magnesium sulfate
. n-Pentane
. Triethylamine
. Silica gel (60, 0.063±0.0200 mm, 70±200 mesh ASTM, Merck)
. 50 mL Round-bottomed flask
. Magnetic stirring bar
. Magnetic stirrer
. Chromatography column
Procedure
1. In a 50 mL round-bottomed flask equipped with a magnetic stirring bar (S,
S)-1,1 -bis(a-acetoxyphenylmethyl)ferrocene (3.00 g) was dissolved in tetra-
0