Page 216 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 216
204 hydrolysis, oxidation and reduction
0
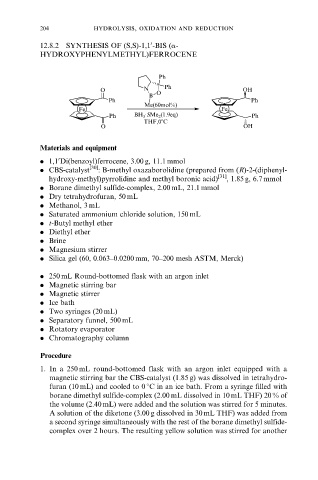
12.8.2 SYNTHESIS OF (S,S)-1,1 -BIS (a-
HYDROXYPHENYLMETHYL)FERROCENE
Ph
O N O Ph OH
B
Ph Ph
Me(60mol%)
Fe Fe
Ph BH ·SMe 2 (1.9eq) Ph
3
THF,08C
O OH
Materials and equipment
0
. 1,1 Di(benzoyl)ferrocene, 3.00 g, 11.1 mmol
. CBS-catalyst [30] : B-methyl oxazaborolidine (prepared from (R)-2-(diphenyl-
hydroxy-methyl)pyrrolidine and methyl boronic acid) [31] , 1.85 g, 6.7 mmol
. Borane dimethyl sulfide-complex, 2.00 mL, 21.1 mmol
. Dry tetrahydrofuran, 50 mL
. Methanol, 3 mL
. Saturated ammonium chloride solution, 150 mL
. t-Butyl methyl ether
. Diethyl ether
. Brine
. Magnesium stirrer
. Silica gel (60, 0.063±0.0200 mm, 70±200 mesh ASTM, Merck)
. 250 mL Round-bottomed flask with an argon inlet
. Magnetic stirring bar
. Magnetic stirrer
. Ice bath
. Two syringes (20 mL)
. Separatory funnel, 500 mL
. Rotatory evaporator
. Chromatography column
Procedure
1. In a 250 mL round-bottomed flask with an argon inlet equipped with a
magnetic stirring bar the CBS-catalyst (1.85 g) was dissolved in tetrahydro-
furan (10 mL) and cooled to 0 8C in an ice bath. From a syringe filled with
borane dimethyl sulfide-complex (2.00 mL dissolved in 10 mL THF) 20 % of
the volume (2.40 mL) were added and the solution was stirred for 5 minutes.
A solution of the diketone (3.00 g dissolved in 30 mL THF) was added from
a second syringe simultaneously with the rest of the borane dimethyl sulfide-
complex over 2 hours. The resulting yellow solution was stirred for another