Page 217 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 217
asymmetric hydrogenation of carbon±carbon double bonds 205
10 minutes at 0 8C and the excess borane dimethyl sulfide-complex was
destroyed by dropwise addition of methanol (caution: gas evolution!).
2. After no further gas evolution could be detected, the reaction mixture was
poured into saturated ammonium chloride solution (150 mL) and trans-
ferred into a separatory funnel. The aqueous layer was extracted with t-
butyl methyl ether (3 70 mL), the combined organic layers were washed
with water (2 100 mL) and brine (100 mL) and dried over magnesium
sulfate. After filtration the solvent was removed using a rotatory evaporator
(bath temperature <30 8C) to give a yellow oil.
3. The crude product was purified by column chromatography (n-pentane:
diethyl ether 1:1) and dried under vacuum yielding (S,S)-1,1 -bis(a-hydro-
0
xyphenyl-methyl)ferrocene (2.93 g, 10.7 mmol, 96 %) as a yellow solid (mp
128±130 8C).
The ee (> 99 %) was determined by HPLC (Daicel 1 OD column, flow
0.6 mL/min, 215 nm, eluent 2-propanol/n-heptane 5/95); (SS and RS): R t
26.53 min, (RR): R t 30.70 min.
1
H-NMR (300 MHz, CDCl 3 ): d 7.27±7.17 (m, 10 H), 5.45 (s, br, 4 H),
4.42 (s, br, 2 H), 4.22 (s, br, 2 H), 4.16 (s, br, 2 H), 4.11 (s, br, 2 H).
13
C-NMR (75 MHz, CDCl 3 ): d 144.08, 128.17, 127.40, 126.19, 93.45,
72.59, 68.10, 67.89, 66.70, 66.66.
0
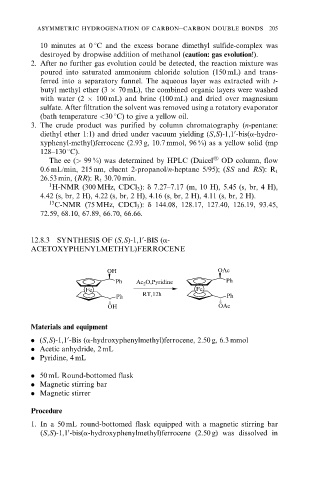
12.8.3 SYNTHESIS OF (S,S)-1,1 -BIS (a-
ACETOXYPHENYLMETHYL)FERROCENE
OH OAc
Ph Ac 2 O,Pyridine Ph
Fe Fe
Ph RT,12h Ph
OH OAc
Materials and equipment
0
. (S,S)-1,1 -Bis (a-hydroxyphenylmethyl)ferrocene, 2.50 g, 6.3 mmol
. Acetic anhydride, 2 mL
. Pyridine, 4 mL
. 50 mL Round-bottomed flask
. Magnetic stirring bar
. Magnetic stirrer
Procedure
1. In a 50 mL round-bottomed flask equipped with a magnetic stirring bar
(S,S)-1,1 -bis(a-hydroxyphenylmethyl)ferrocene (2.50 g) was dissolved in
0