Page 212 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 212
200 hydrolysis, oxidation and reduction
at ÿ20 8C. The cold bath was removed immediately and the reaction mixture
was warmed to room temperature.
2. After stirring at room temperature for 20 minutes, the reaction mixture was
cooled to 0 8C and transferred via a cannula into saturated aqueous NaHCO 3
(15 mL). Saturated aqueous sodium potassium tartrate was added (15 mL).
3. The dichloromethane was removed by rotatory evaporation and dry ether
(30 mL) was added. The mixture was stirred vigorously for 15 minutes and
then acidified with 1 N HCl (15 mL).
4. The organic layer was separated, and the aqueous layer was extracted with
ether (3 20 mL). The combined organic layer was washed with brine, dried
over magnesium sulfate, filtered and concentrated using a rotatory evapor-
ator. The crude orange solid was recrystallized from hot ethanol to give the
( p S, p S)-1,1 -bis(diphenylphosphino)-2,2 -di-g-pentylferrocene [(S,S)-3-Pt-
0
0
FerroPHOS] as yellow crystals (519 mg, 45.0 %).
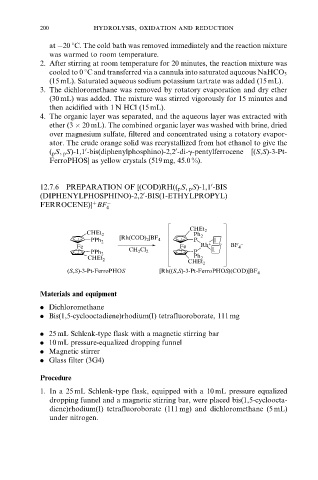
12.7.6 PREPARATION OF [(COD)RH(( p S, p S)-1,1 -BIS
0
(DIPHENYLPHOSPHINO)-2,2 -BIS(1-ETHYLPROPYL)
0
FERROCENE)] BF ÿ
4
CHEt 2
CHEt 2
Ph 2
[Rh(COD) 2 ]BF 4 P
PPh 2 −
Fe Fe Rh + BF 4
CH 2 Cl 2 P
PPh 2
Ph 2
CHEt 2
CHEt 2
(S,S)-3-Pt-FerroPHOS [Rh((S,S)-3-Pt-FerroPHOS)(COD)]BF 4
Materials and equipment
. Dichloromethane
. Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate, 111 mg
. 25 mL Schlenk-type flask with a magnetic stirring bar
. 10 mL pressure-equalized dropping funnel
. Magnetic stirrer
. Glass filter (3G4)
Procedure
1. In a 25 mL Schlenk-type flask, equipped with a 10 mL pressure equalized
dropping funnel and a magnetic stirring bar, were placed bis(1,5-cycloocta-
diene)rhodium(I) tetrafluoroborate (111 mg) and dichloromethane (5 mL)
under nitrogen.