Page 210 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 210
198 hydrolysis, oxidation and reduction
. Separatory funnel, 250 mL
. Rotatory evaporator
Procedure
1. (R,R)-1,1 -Bis[a-(dimethylamino)propyl]ferrocene (5.10 g) was placed in a
0
250 mL round-bottomed flask equipped with a magnetic stirring bar under
nitrogen; dry diethyl ether (22 mL) was then added. To the mixture was
added dropwise n-BuLi in hexanes (1.68 M, 34.0 mL) within 10 minutes at
room temperature. After 30 minutes the colour of the mixture changed from
yellow to red.
2. After 6 hours, chlorodiphenylphosphine (18 mL) was added over 2 hours with
the help of a syringe pump. After the addition was complete, the resulting
suspension was stirred at room temperature for 3 hours and aqueous sodium
bicarbonate was slowly added to hydrolyse the excess chlorodiphenylpho-
sphine with cooling in an ice bath.
3. The reaction mixture was extracted with diethyl ether (3 30 mL). The
combined organic layer was washed with brine, dried over magnesium sulfate,
filtered and concentrated using a rotatory evaporator. The resulting residue
was chromatographed (eluent: n-hexane±ethyl acetate, 97:3) on silica gel pre-
deactivated with triethylamine: n-hexane (2:98) to afford the (R, R, p S, p S)-
0
0
1,1 -bis[a-(dimethylamino)propyl]-2,2 -bis(diphenylphosphino)ferrocene as
an orange solid (8.07 g, 78.0 %).
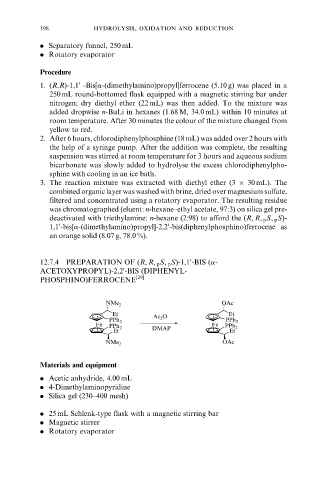
12.7.4 PREPARATION OF (R, R, p S, p S)-1,1 -BIS (a-
0
ACETOXYPROPYL)-2,2 -BIS (DIPHENYL-
0
PHOSPHINO)FERROCENE [29]
OAc
NMe 2
Et Et
Ac 2 O
PPh 2 PPh 2
Fe Fe
PPh 2 DMAP PPh 2
Et Et
OAc
NMe 2
Materials and equipment
. Acetic anhydride, 4.00 mL
. 4-Dimethylaminopyridine
. Silica gel (230±400 mesh)
. 25 mL Schlenk-type flask with a magnetic stirring bar
. Magnetic stirrer
. Rotatory evaporator