Page 206 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 206
194 hydrolysis, oxidation and reduction
enzyme-promoted reductions which have more limited scope with respect to
substrates. The enantioselective hydrogenations have been applied to the
synthesis of natural products of biological interest [25] .
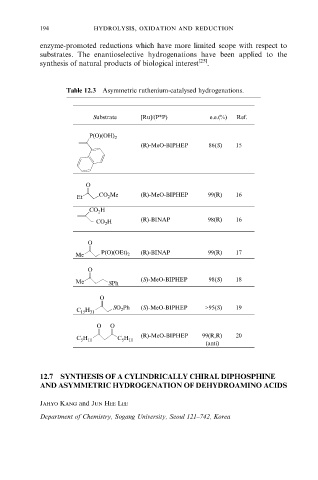
Table 12.3 Asymmetric ruthenium-catalysed hydrogenations.
Substrate [Ru]/(P*P) e.e.(%) Ref.
P(O)(OH) 2
(R)-MeO-BIPHEP 86(S) 15
O
Et CO 2 Me (R)-MeO-BIPHEP 99(R) 16
CO 2 H
CO 2 H (R)-BINAP 98(R) 16
O
Me P(O)(OEt) 2 (R)-BINAP 99(R) 17
O
(S)-MeO-BIPHEP 98(S) 18
Me SPh
O
SO 2 Ph (S)-MeO-BIPHEP >95(S) 19
C 15 H 31
O O
(R)-MeO-BIPHEP 99(R,R) 20
C 5 H 11 C 5 H 11
(anti)
12.7 SYNTHESIS OF A CYLINDRICALLY CHIRAL DIPHOSPHINE
AND ASYMMETRIC HYDROGENATION OF DEHYDROAMINO ACIDS
Jahyo Kang and Jun Hee Lee
Department of Chemistry, Sogang University, Seoul 121±742, Korea