Page 201 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 201
asymmetric hydrogenation of carbon±carbon double bonds 189
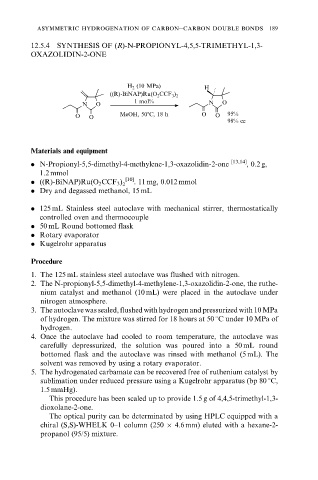
12.5.4 SYNTHESIS OF (R)-N-PROPIONYL-4,5,5-TRIMETHYL-1,3-
OXAZOLIDIN-2-ONE
H 2 (10 MPa) H
((R)-BiNAP)Ru(O 2 CCF 3 ) 2
1 mol% N O
N O
O O MeOH, 508C, 18 h O O 95%
98% ee
Materials and equipment
. N-Propionyl-5,5-dimethyl-4-methylene-1,3-oxazolidin-2-one [13,14] , 0.2 g,
1.2 mmol
. ((R)-BiNAP)Ru(O 2 CCF 3 ) 2 [10] . 11 mg, 0.012 mmol
. Dry and degassed methanol, 15 mL
. 125 mL Stainless steel autoclave with mechanical stirrer, thermostatically
controlled oven and thermocouple
. 50 mL Round bottomed flask
. Rotary evaporator
. Kugelrohr apparatus
Procedure
1. The 125 mL stainless steel autoclave was flushed with nitrogen.
2. The N-propionyl-5,5-dimethyl-4-methylene-1,3-oxazolidin-2-one, the ruthe-
nium catalyst and methanol (10 mL) were placed in the autoclave under
nitrogen atmosphere.
3. The autoclave was sealed, flushed with hydrogen and pressurized with 10 MPa
of hydrogen. The mixture was stirred for 18 hours at 50 8C under 10 MPa of
hydrogen.
4. Once the autoclave had cooled to room temperature, the autoclave was
carefully depressurized, the solution was poured into a 50 mL round
bottomed flask and the autoclave was rinsed with methanol (5 mL). The
solvent was removed by using a rotary evaporator.
5. The hydrogenated carbamate can be recovered free of ruthenium catalyst by
sublimation under reduced pressure using a Kugelrohr apparatus (bp 80 8C,
1.5 mmHg).
This procedure has been scaled up to provide 1.5 g of 4,4,5-trimethyl-1,3-
dioxolane-2-one.
The optical purity can be determinated by using HPLC equipped with a
chiral (S,S)-WHELK 0±1 column (250 4.6 mm) eluted with a hexane-2-
propanol (95/5) mixture.