Page 198 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 198
186 hydrolysis, oxidation and reduction
Note that, in contrast, the reactions using [(COD) Rh ((S, S)Me-BPE)] or
[(COD)Rh((R, R) Me-DuPHOS)] complexes can be performed at atmospheric
pressure of hydrogen which avoids the use of heavy-duty hydrogenation ap-
paratus.
12.5 HYDROGENATION OF ENOL CARBONATES AND 4-
METHYLENE-N-ACYLOXAZOLIDINONE USING [Rh((R)-BiNAP)]
COMPLEXES
P.H. Dixneuf, C. Bruneau and P. Le Gendre
UMR6509, Organometalliques et Catalyse: Chimie et Electrochimie Moleculaire, Uni-
versite de Rennes 1, Laboratoire de Chimie de Coordination Organique, Campus de
Beaulieu, Avenue du ge Âne Âral Leclerc, 35042 Rennes Cedex, Tel: 33 (0)2 99 28 62 80,
Fax: 33 (0)2 99 28 69 39, e-mail: pierre.dixneuf@univ-rennes 1. fr
H R H R
R R
O O HO OH
O
ÿ
ÿ
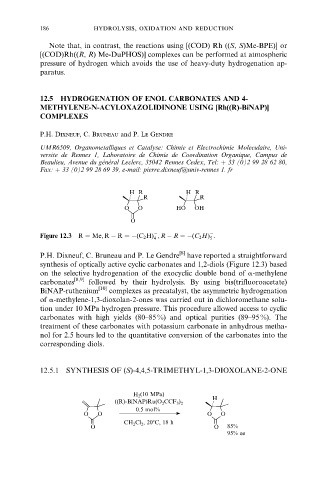
Figure 12.3 R Me, R ÿ R ÿ(C 2 H) , R ÿ R ÿ(C 2 H) .
4
5
P.H. Dixneuf, C. Bruneau and P. Le Gendre [8] have reported a straightforward
synthesis of optically active cyclic carbonates and 1,2-diols (Figure 12.3) based
on the selective hydrogenation of the exocyclic double bond of a-methylene
carbonates [8,9] followed by their hydrolysis. By using bis(trifluoroacetate)
BiNAP-ruthenium [10] complexes as precatalyst, the asymmetric hydrogenation
of a-methylene-1,3-dioxolan-2-ones was carried out in dichloromethane solu-
tion under 10 MPa hydrogen pressure. This procedure allowed access to cyclic
carbonates with high yields (80±85 %) and optical purities (89±95 %). The
treatment of these carbonates with potassium carbonate in anhydrous metha-
nol for 2.5 hours led to the quantitative conversion of the carbonates into the
corresponding diols.
12.5.1 SYNTHESIS OF (S)-4,4,5-TRIMETHYL-1,3-DIOXOLANE-2-ONE
H 2 (10 MPa)
H
((R)-BiNAP)Ru(O 2 CCF 3 ) 2
0.5 mol%
O O O O
CH 2 Cl 2 , 208C, 18 h
O O 85%
95% ee