Page 196 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 196
184 hydrolysis, oxidation and reduction
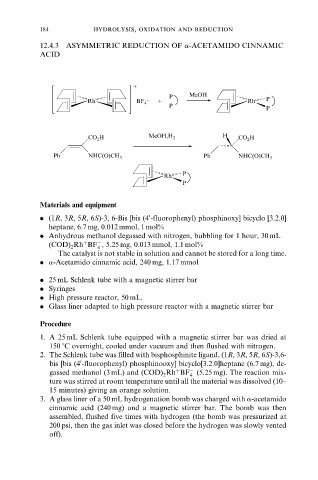
12.4.3 ASYMMETRIC REDUCTION OF a-ACETAMIDO CINNAMIC
ACID
+
P MeOH
Rh BF 4 − + Rh P
P P
CO 2 H MeOH,H 2 H CO 2 H
Ph NHC(O)CH 3 Ph NHC(O)CH 3
P
Rh
P
Materials and equipment
. (1R, 3R, 5R, 6S)-3, 6-Bis [bis (4 -fluorophenyl) phosphinoxy] bicyclo [3.2.0]
0
heptane, 6.7 mg, 0.012 mmol, 1 mol%
. Anhydrous methanol degassed with nitrogen, bubbling for 1 hour, 30 mL
ÿ
(COD) Rh BF , 5.25 mg, 0.013 mmol, 1.1 mol%
2
4
The catalyst is not stable in solution and cannot be stored for a long time.
. a-Acetamido cinnamic acid, 240 mg, 1.17 mmol
. 25 mL Schlenk tube with a magnetic stirrer bar
. Syringes
. High pressure reactor, 50 mL.
. Glass liner adapted to high pressure reactor with a magnetic stirrer bar
Procedure
1. A 25 mL Schlenk tube equipped with a magnetic stirrer bar was dried at
150 8C overnight, cooled under vacuum and then flushed with nitrogen.
2. The Schlenk tube was filled with bisphosphinite ligand, (1R, 3R, 5R, 6S)-3,6-
bis [bis (4 -fluorophenyl) phosphinooxy] bicyclo[3.2.0]heptane (6.7 mg), de-
0
gassed methanol (3 mL) and (COD) Rh BF (5.25 mg). The reaction mix-
ÿ
2
4
ture was stirred at room temperature until all the material was dissolved (10±
15 minutes) giving an orange solution.
3. A glass liner of a 50 mL hydrogenation bomb was charged with a-acetamido
cinnamic acid (240 mg) and a magnetic stirrer bar. The bomb was then
assembled, flushed five times with hydrogen (the bomb was pressurized at
200 psi, then the gas inlet was closed before the hydrogen was slowly vented
off).