Page 200 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 200
188 hydrolysis, oxidation and reduction
. 50 mL Round bottomed flask with a magnetic stirrer bar
. Reflux condenser
. Magnetic stirrer plate with thermostatically controlled oil bath and therm-
ometer
. Rotary evaporator
. Kugelrohr apparatus
Procedure
1. 4,4,5-Trimethyl-1,3-dioxolane-2-one (0.17 g), potassium carbonate (0.27 g)
and methanol (10 mL) were placed in 50 mL round bottomed flask equipped
with a magnetic stirrer bar and a reflux condenser. The mixture was then
stirred at 60 8C for 2.5 hours.
2. The solvent was removed by using a rotary evaporator. The solution was
dissolved in a saturated solution of NH 4 Cl and extracted with diethyl ether.
After the solution was dried with magnesium sulfate, the diethyl ether was
removed by using a rotary evaporator.
3. It is noteworthy that this diol has been used as ligand in the molybdenum-
mediated kinetic resolution of oxiranes [11] .
12.5.3 PREPARATION OF OPTICALLY ACTIVE N-
ACYLOXAZOLIDINONES
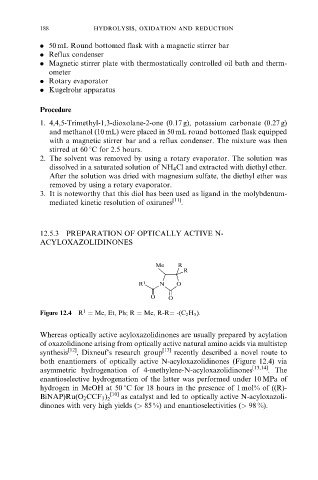
Me R
R
R 1 N O
O O
1
Figure 12.4 R Me, Et, Ph; R Me, R-R -(C 2 H 5 ).
Whereas optically active acyloxazolidinones are usually prepared by acylation
of oxazolidinone arising from optically active natural amino acids via multistep
synthesis [12] , Dixneuf's research group [13] recently described a novel route to
both enantiomers of optically active N-acyloxazolidinones (Figure 12.4) via
asymmetric hydrogenation of 4-methylene-N-acyloxazolidinones [13,14] . The
enantioselective hydrogenation of the latter was performed under 10 MPa of
hydrogen in MeOH at 50 8C for 18 hours in the presence of 1 mol% of ((R)-
BiNAP)Ru(O 2 CCF 3 ) 2 [10] as catalyst and led to optically active N-acyloxazoli-
dinones with very high yields (> 85 %) and enantioselectivities (> 98 %).