Page 197 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 197
asymmetric hydrogenation of carbon±carbon double bonds 185
4. The solution of the catalyst (formed in situ) was added via a syringe (3 mL)
through the solvent port equipped with a septum, and the mixture stirred.
5. The hydrogenation bomb was pressurized to 200 psi of hydrogen (14 atm).
The reaction performed at room temperature was complete after 3 hours
(followed by GC/MS). N-Acetyl-l-phenylalanine was obtained in quantita-
tive yield.
1
The ee (91 %) was determined by chiral HPLC (Chiralpak AD, Hexane±
IPA±TFA, 89 %±10 %±1 %, sample dissolved in IPA) R t (R)-enantiomer:
11.9 min, R t (S)-enantiomer: 14.3 min.
1 H NMR (200 MHz, DMSO): d 8.22 (d, J 8.2 Hz, 1H, NH); 7.24 (m, 5H,
Ph); 4.40 (m, 1H, CH); 3.02 (dd, J 13.8 Hz, J 5.0 Hz, 1H, CH a H b ); 2.83 (dd,
J 13.8 Hz, J 9.5 Hz, 1H, CH a H b ); 1.78 (s, 3H, CO-CH 3 ).
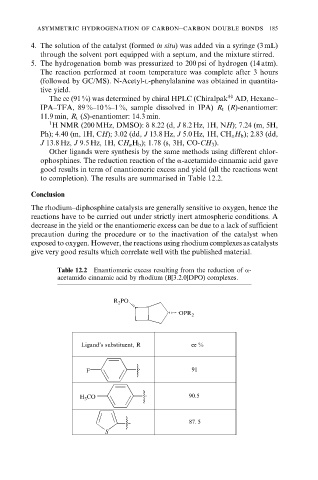
Other ligands were synthesis by the same methods using different chlor-
ophosphines. The reduction reaction of the a-acetamido cinnamic acid gave
good results in term of enantiomeric excess and yield (all the reactions went
to completion). The results are summarised in Table 12.2.
Conclusion
The rhodium±diphosphine catalysts are generally sensitive to oxygen, hence the
reactions have to be carried out under strictly inert atmospheric conditions. A
decrease in the yield or the enantiomeric excess can be due to a lack of sufficient
precaution during the procedure or to the inactivation of the catalyst when
exposed to oxygen. However, the reactions using rhodium complexes as catalysts
give very good results which correlate well with the published material.
Table 12.2 Enantiomeric excess resulting from the reduction of a-
acetamido cinnamic acid by rhodium (B[3.2.0]DPO) complexes.
R 2 PO
OPR 2
Ligand’s substituent, R ee %
F 91
H 3 CO 90.5
87. 5
S