Page 193 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 193
asymmetric hydrogenation of carbon±carbon double bonds 181
. Cycloocta-1,5-diene, 1.30 g, 12 mmol, 1.2 eq
. Tetrafluoroboric acid±diethyl ether complex (HBF 4 :OEt 2 ) in diethyl-
ether, 54 %, 3.00 g, 2.52 mL, 10 mmol, 1 eq, diluted with tetrahydrofuran,
5 mL
. Dry diethyl ether
. 100 mL Schlenk tube with a magnetic stirrer bar.
. Condenser.
. Magnetic stirrer hot plate with a thermostatically controlled oil-bath and
thermometer
. Sinter funnel with an inert gas inlet
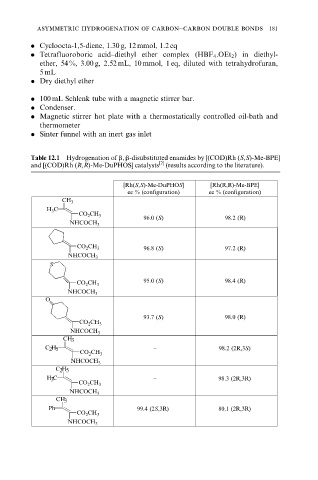
Table 12.1 Hydrogenation of b, b-disubstituted enamides by [(COD)Rh (S,S)-Me-BPE]
and [(COD)Rh (R,R)-Me-DuPHOS] catalysts [2] (results according to the literature).
[Rh(S,S)-Me-DuPHOS] [Rh(R,R)-Me-BPE]
ee % (configuration) ee % (configuration)
CH 3
H 3 C
CO 2 CH 3
96.0 (S) 98.2 (R)
NHCOCH 3
CO 2 CH 3 96.8 (S) 97.2 (R)
NHCOCH 3
S
95.0 (S) 98.4 (R)
CO 2 CH 3
NHCOCH 3
O
93.7 (S) 98.0 (R)
CO 2 CH 3
NHCOCH 3
CH 3
C 2 H 5 − 98.2 (2R,3S)
CO 2 CH 3
NHCOCH 3
C 2 H 5
H 3 C − 98.3 (2R,3R)
CO 2 CH 3
NHCOCH 3
CH 3
Ph 99.4 (2S,3R) 80.1 (2R,3R)
CO 2 CH 3
NHCOCH 3