Page 189 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 189
asymmetric hydrogenation of carbon±carbon double bonds 177
H
L* n M
H
C H H
C L* n M
H
L* n M
H
L* n M
C C H 2
H H
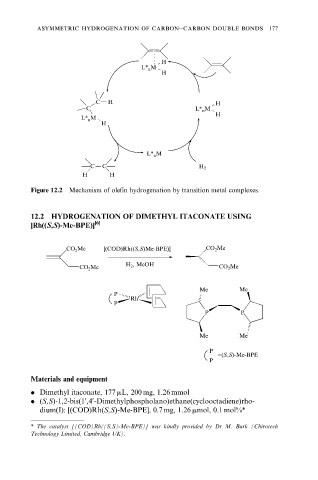
Figure 12.2 Mechanism of olefin hydrogenation by transition metal complexes.
12.2 HYDROGENATION OF DIMETHYL ITACONATE USING
[Rh((S,S)-Me-BPE)] [6]
CO 2 Me [(COD)Rh((S,S)Me-BPE)] CO 2 Me
H 2 , MeOH
CO 2 Me CO 2 Me
Me Me
P
Rh
P
P P
Me Me
P
=(S,S)-Me-BPE
P
Materials and equipment
. Dimethyl itaconate, 177 mL, 200 mg, 1.26 mmol
. (S,S)-1,2-bis(1 ,4 -Dimethylphospholano)ethane(cyclooctadiene)rho-
0
0
dium(I): [(COD)Rh(S,S)-Me-BPE], 0.7 mg, 1.26 mmol, 0.1 mol%*
* The catalyst [(COD)Rh((S,S)-Me-BPE)] was kindly provided by Dr M. Burk (Chirotech
Technology Limited, Cambridge UK).