Page 194 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 194
182 hydrolysis, oxidation and reduction
Procedure
1. A 100 mL Schlenk flask equipped with a magnetic stirrer bar and a con-
denser was dried at 150 8C overnight, cooled under vacuum and then flushed
with nitrogen.
2. The Schlenk tube was filled with [(COD)Rh(acac)] (3.1 g) and cycloocta-1,5-
diene (1.30 g) which were dissolved in 15 mL of dry tetrahydrofuran. To this
orange mixture, the solution of HBF 4 :OEt 2 in tetrahydrofuran (7.52 mL)
was added. A brown precipitate appeared, giving a viscous solution, which
was diluted with 40 mL additional tetrahydrofuran to allow the reaction to
stir efficiently.
3. The orange solution was heated (80 8C) to reflux under nitrogen for 30
minutes.
4. The brown solution was cooled to room temperature. The brown powder
was filtered under nitrogen using a sinter funnel with an inert gas inlet, and
then washed with dry diethyl ether (3 5 mL).
5. The [(COD) Rh BF ] complex obtained was used as the catalyst precursor
ÿ
2
4
for hydrogenation without further purification.
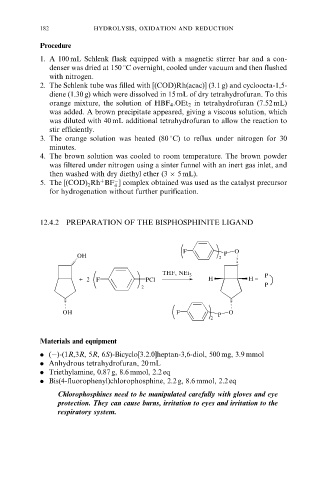
12.4.2 PREPARATION OF THE BISPHOSPHINITE LIGAND
F O
OH 2 P
THF, NEt 3 P
+ 2 F PCl H H =
P
2
OH F P O
2
Materials and equipment
. (ÿ)-(1R,3R, 5R, 6S)-Bicyclo[3.2.0]heptan-3,6-diol, 500 mg, 3.9 mmol
. Anhydrous tetrahydrofuran, 20 mL
. Triethylamine, 0.87 g, 8.6 mmol, 2.2 eq
. Bis(4-fluorophenyl)chlorophosphine, 2.2 g, 8.6 mmol, 2.2 eq
Chlorophosphines need to be manipulated carefully with gloves and eye
protection. They can cause burns, irritation to eyes and irritation to the
respiratory system.