Page 191 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 191
asymmetric hydrogenation of carbon±carbon double bonds 179
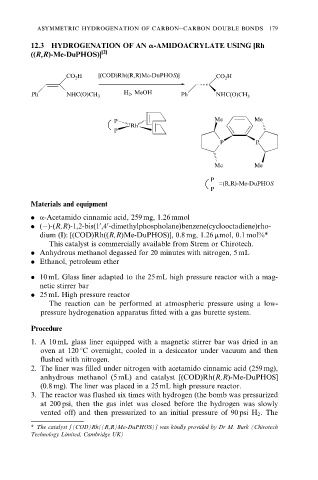
12.3 HYDROGENATION OF AN a-AMIDOACRYLATE USING [Rh
((R,R)-Me-DuPHOS)] [2]
CO 2 H [(COD)Rh((R,R)Me-DuPHOS)] CO 2 H
Ph NHC(O)CH 3 H 2 , MeOH Ph NHC(O)CH 3
P Me Me
Rh
P
P P
Me Me
P
=(R,R)-Me-DuPHOS
P
Materials and equipment
. a-Acetamido cinnamic acid, 259 mg, 1.26 mmol
. (ÿ)-(R,R)-1,2-bis(1 ,4 -dimethylphospholane)benzene(cyclooctadiene)rho-
0
0
dium (I): [(COD)Rh((R,R)Me-DuPHOS)], 0.8 mg, 1.26 mmol, 0.1 mol%*
This catalyst is commercially available from Strem or Chirotech.
. Anhydrous methanol degassed for 20 minutes with nitrogen, 5 mL
. Ethanol, petroleum ether
. 10 mL Glass liner adapted to the 25 mL high pressure reactor with a mag-
netic stirrer bar
. 25 mL High pressure reactor
The reaction can be performed at atmospheric pressure using a low-
pressure hydrogenation apparatus fitted with a gas burette system.
Procedure
1. A 10 mL glass liner equipped with a magnetic stirrer bar was dried in an
oven at 120 8C overnight, cooled in a desiccator under vacuum and then
flushed with nitrogen.
2. The liner was filled under nitrogen with acetamido cinnamic acid (259 mg),
anhydrous methanol (5 mL) and catalyst [(COD)Rh(R,R)-Me-DuPHOS]
(0.8 mg). The liner was placed in a 25 mL high pressure reactor.
3. The reactor was flushed six times with hydrogen (the bomb was pressurized
at 200 psi, then the gas inlet was closed before the hydrogen was slowly
vented off) and then pressurized to an initial pressure of 90 psi H 2 . The
* The catalyst [(COD)Rh((R,R)Me-DuPHOS)] was kindly provided by Dr M. Burk (Chirotech
Technology Limited, Cambridge UK)