Page 202 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 202
190 hydrolysis, oxidation and reduction
12.6 ENANTIOSELECTIVE RUTHENIUM-CATALYZED
HYDROGENATION OF VINYLPHOSPHONIC ACIDS
Virginie Ratovelomanana-Vidal, Jean-Pierre GenE Ã t
Laboratoire de Synthe Áse Organique Se Âlective & Produits Naturels, Ecole Nationale Supe Âr-
ieure de Chimie de Paris, 11, rue Pierre & Marie Curie 75231 Paris cedex 05 France, Tel: 01
44 27 67 43 fax: 01 44 07 10 62, e-mail:genet@ext.jussieu.fr
12.6.1 SYNTHESIS OF CHIRAL RU(II) CATALYSTS
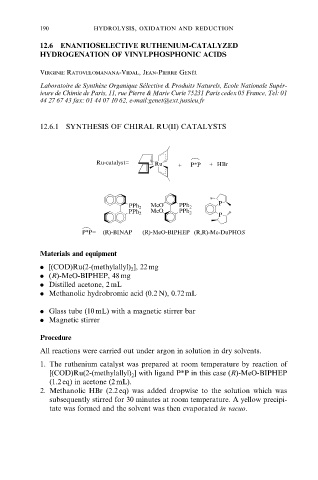
Ru-catalyst= Ru + P*P + HBr
MeO P
PPh 2 PPh 2
MeO
PPh 2 PPh 2
P
P*P= (R)-BINAP (R)-MeO-BIPHEP (R,R)-Me-DuPHOS
Materials and equipment
. [(COD)Ru(2-(methylallyl) ], 22 mg
2
. (R)-MeO-BIPHEP, 48 mg
. Distilled acetone, 2 mL
. Methanolic hydrobromic acid (0.2 N), 0.72 mL
. Glass tube (10 mL) with a magnetic stirrer bar
. Magnetic stirrer
Procedure
All reactions were carried out under argon in solution in dry solvents.
1. The ruthenium catalyst was prepared at room temperature by reaction of
[(COD)Ru(2-(methylallyl) ] with ligand P*P in this case (R)-MeO-BIPHEP
2
(1.2 eq) in acetone (2 mL).
2. Methanolic HBr (2.2 eq) was added dropwise to the solution which was
subsequently stirred for 30 minutes at room temperature. A yellow precipi-
tate was formed and the solvent was then evaporated in vacuo.