Page 203 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 203
asymmetric hydrogenation of carbon±carbon double bonds 191
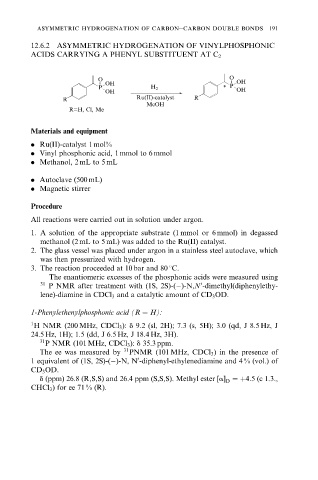
12.6.2 ASYMMETRIC HYDROGENATION OF VINYLPHOSPHONIC
ACIDS CARRYING A PHENYL SUBSTITUENT AT C 2
O O
OH OH
P H 2 * P
OH OH
R Ru(II)-catalyst R
MeOH
R=H, Cl, Me
Materials and equipment
. Ru(II)-catalyst 1 mol%
. Vinyl phosphonic acid, 1 mmol to 6 mmol
. Methanol, 2 mL to 5 mL
. Autoclave (500 mL)
. Magnetic stirrer
Procedure
All reactions were carried out in solution under argon.
1. A solution of the appropriate substrate (1 mmol or 6 mmol) in degassed
methanol (2 mL to 5 mL) was added to the Ru(II) catalyst.
2. The glass vessel was placed under argon in a stainless steel autoclave, which
was then pressurized with hydrogen.
3. The reaction proceeded at 10 bar and 80 8C.
The enantiomeric excesses of the phosphonic acids were measured using
31 P NMR after treatment with (1S, 2S)-(ÿ)-N,N -dimethyl(diphenylethy-
0
lene)-diamine in CDCl 3 and a catalytic amount of CD 3 OD.
1-Phenylethenylphosphonic acid (R H):
1 H NMR (200 MHz, CDCl 3 ): d 9.2 (sl, 2H); 7.3 (s, 5H); 3.0 (qd, J 8.5 Hz, J
24.5 Hz, 1H); 1.5 (dd, J 6.5 Hz, J 18.4 Hz, 3H).
31 P NMR (101 MHz, CDCl 3 ): d 35.3 ppm.
The ee was measured by 31 PNMR (101 MHz, CDCl 3 ) in the presence of
1 equivalent of (1S, 2S)-(ÿ)-N, N -diphenyl-ethylenediamine and 4 % (vol.) of
0
CD 3 OD.
d (ppm) 26.8 (R,S,S) and 26.4 ppm (S,S,S). Methyl ester [a] 4:5 (c 1.3.,
D
CHCl 3 ) for ee 71 % (R).