Page 208 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 208
196 hydrolysis, oxidation and reduction
4. The residue was chromatographed on silica gel (eluent: n-hexane±ethyl
acetate, 3:1) to afford the (R,R)-1,1 -bis(a-hydroxypropyl)ferrocene as an
0
orange solid (9.83 g, 95 %).
The ee (99.9 %) was determined by HPLC (Daicel Chiralcel OJ column,
eluent 2-propanol-n-hexane 2:98, flow 0.5 mL/min); (S,S)-enantiomer: R t
20.0 min, (R,S)-meso isomer: R t 24.8 min, (R,R)-enantiomer: R t 35.6 min;
the (S,S)-isomer was not detected); (R,R)-isomer: (R,S)-meso isomer
98.3:1.7.
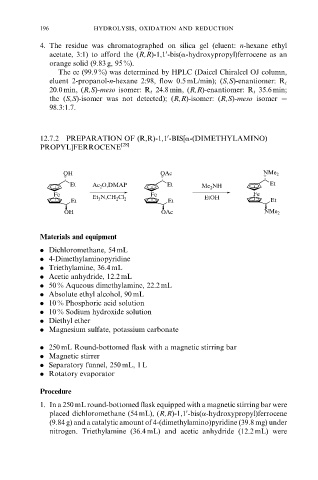
12.7.2 PREPARATION OF (R,R)-1,1 -BIS[a-(DIMETHYLAMINO)
0
PROPYL]FERROCENE [28]
OH OAc NMe 2
Et Ac 2 O,DMAP Et Me 2 NH Et
Fe Fe Fe
Et 3 N,CH 2 Cl 2 EtOH
Et Et Et
OH OAc NMe 2
Materials and equipment
. Dichloromethane, 54 mL
. 4-Dimethylaminopyridine
. Triethylamine, 36.4 mL
. Acetic anhydride, 12.2 mL
. 50 % Aqueous dimethylamine, 22.2 mL
. Absolute ethyl alcohol, 90 mL
. 10 % Phosphoric acid solution
. 10 % Sodium hydroxide solution
. Diethyl ether
. Magnesium sulfate, potassium carbonate
. 250 mL Round-bottomed flask with a magnetic stirring bar
. Magnetic stirrer
. Separatory funnel, 250 mL, 1 L
. Rotatory evaporator
Procedure
1. In a 250 mL round-bottomed flask equipped with a magnetic stirring bar were
placed dichloromethane (54 mL), (R,R)-1,1 -bis(a-hydroxypropyl)ferrocene
0
(9.84 g) and a catalytic amount of 4-(dimethylamino)pyridine (39.8 mg) under
nitrogen. Triethylamine (36.4 mL) and acetic anhydride (12.2 mL) were