Page 209 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 209
asymmetric hydrogenation of carbon±carbon double bonds 197
added to the mixture successively at 0 8C, and the resulting mixture was
stirred at room temperature for 8 hours.
2. Cold water (ice±water, 50 mL) was added, and the mixture was extracted
with dichloromethane (3 30 mL). The combined extracts were dried over
magnesium sulfate, filtered and concentrated using a rotatory evaporator to
afford the diacetate as a dark-brown residue.
3. To the residue in a 250 mL round-bottomed flask equipped with a magnetic
stirring bar were added 50 % aqueous dimethylamine (22.2 mL) and absolute
ethyl alcohol (90 mL), the mixture was stirred at room temperature for 48
hours. During this time, an orange solid was formed.
4. The solvent was removed using a rotatory evaporator, and the resulting
residue was diluted with ether (50 mL). The diamine was extracted with
10 % phosphoric acid (3 15 mL), after which the aqueous layer was made
alkaline (pH 9) with 10 % sodium hydroxide solution (100 mL). The resulting
mixture was extracted with ether (5 50 mL). The combined ethereal extracts
were dried over anhydrous potassium carbonate, filtered and concentrated in
vacuo to afford the pure (R,R)-1,1 -bis [a-(dimethylamino) propyl]ferrocene
0
as an orange solid (11.4 g, 98.0 %).
0
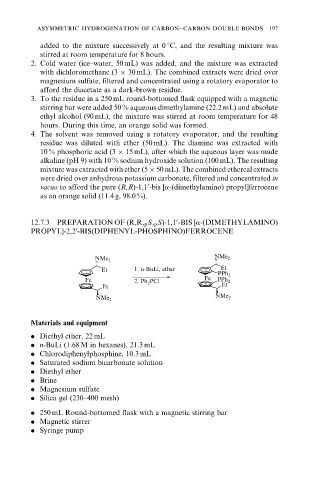
12.7.3 PREPARATION OF (R,R, p S, p S)-1,1 -BIS [a-(DIMETHYLAMINO)
PROPYL]-2,2 -BIS(DIPHENYL-PHOSPHINO)FERROCENE
0
NMe 2 NMe 2
Et 1. n-BuLi, ether Et
PPh 2
Fe PCl Fe PPh 2
2. Ph 2 Et
Et
NMe 2 NMe 2
Materials and equipment
. Diethyl ether, 22 mL
. n-BuLi (1.68 M in hexanes), 21.3 mL
. Chlorodiphenylphosphine, 10.3 mL
. Saturated sodium bicarbonate solution
. Diethyl ether
. Brine
. Magnesium sulfate
. Silica gel (230±400 mesh)
. 250 mL Round-bottomed flask with a magnetic stirring bar
. Magnetic stirrer
. Syringe pump