Page 219 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 219
asymmetric hydrogenation of carbon±carbon double bonds 207
hydrofuran and dimethylamine (10 mL, 40 % in water) was added. Then
water was added dropwise until a yellow solid started to precipitate, where-
upon the solid was dissolved again by addition of tetrahydrofuran and the
suspension was stirred for 12 hours at room temperature.
2. The tetrahydrofuran was removed under vacuum (1 mmHg), water was
added (50 mL) and the solution was transferred into a separatory funnel.
After extraction with t-butyl methyl ether (3 100 mL) the combined
organic layers were washed with water (2 50 mL) and brine (2 50 mL)
and dried over magnesium sulfate. After filtration the solvent was removed
using a rotatory evaporator to give a yellow oil.
3. The crude product was purified by column chromatography (n-pentane: di-
ethyl ether 3:1, 1 % triethylamine) and dried undervacuum yielding (S, S)-1,1 -
0
bis(a-N,N-dimethylaminophenylmethyl)ferrocene (2.45 g, 5.41 mmol, 87 %)
as a brown solid (48±49 8C).
1 H-NMR (300 MHz, CDCl 3 ): d 7.43±7.28 (m, 10 H), 3.89±3.88 (m, 2 H),
3.61 (s, br, 2 H), 3.56±3.55 (m, 2 H), 3.50±3.49 (m, 4 H), 1.98 (s, 12 H).
13
C-NMR (75 MHz, CDCl 3 ): d 143.34, 128.40, 127.97, 127.00, 90.38,
72.39, 71,39, 70.07, 67.74, 67.67, 44.48.
0
0
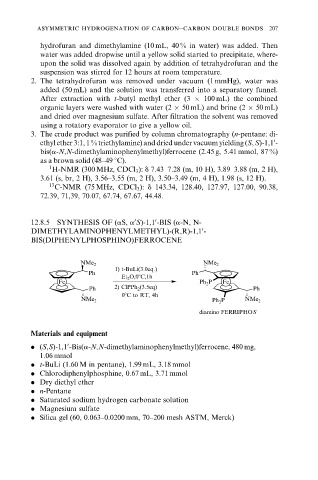
12.8.5 SYNTHESIS OF (aS, a S)-1,1 -BIS (a-N, N-
DIMETHYLAMINOPHENYLMETHYL)-(R,R)-1,1 -
0
BIS(DIPHENYLPHOSPHINO)FERROCENE
NMe 2 NMe 2
1) t-BuLi(3.0eq.)
Ph Ph
Et 2 O,08C,1h
Fe Ph 2 P Fe
Ph 2) ClPPh 2 (3.5eq) Ph
08C to RT, 4h
Ph 2 P
NMe 2 NMe 2
diamino FERRIPHOS
Materials and equipment
. (S,S)-1,1 -Bis(a-N,N-dimethylaminophenylmethyl)ferrocene, 480 mg,
0
1.06 mmol
. t-BuLi (1.60 M in pentane), 1.99 mL, 3.18 mmol
. Chlorodiphenylphosphine, 0.67 mL, 3.71 mmol
. Dry diethyl ether
. n-Pentane
. Saturated sodium hydrogen carbonate solution
. Magnesium sulfate
. Silica gel (60, 0.063±0.0200 mm, 70±200 mesh ASTM, Merck)